Space enterococcus faecium LCT (Liquid-based Cytology Test )-EF301
A technology of LCT-EF301, Enterococcus faecium, applied in the field of microorganisms, can solve the problems that have not been reported, limitations and other issues.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
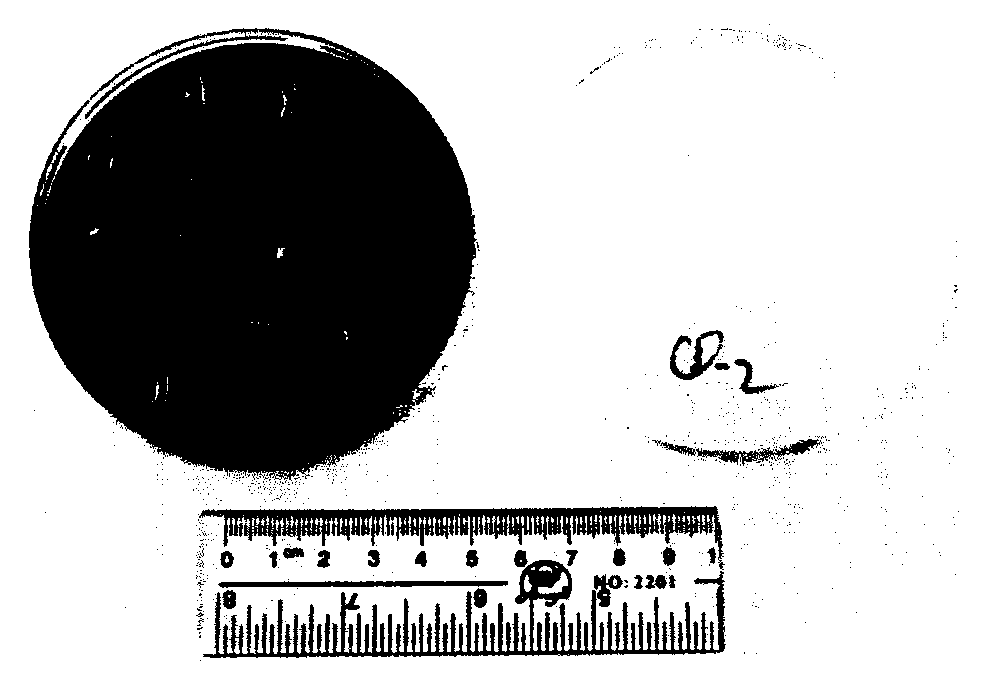
[0018] Embodiment 1, hemolysis test of Enterococcus faecium LCT-E F301
[0019] The hemolysis test adopts spotting method. The strains are spot-planted on the blood agar medium, kept at a constant temperature of 37°C, and cultured upside down. As a result, there is no hemolysis pattern around the colony of Enterococcus faecium LCT-E F301, see figure 1 . It can be seen from the figure that Enterococcus faecium LCT-EF301 has no hemolysis phenomenon.
Embodiment 2
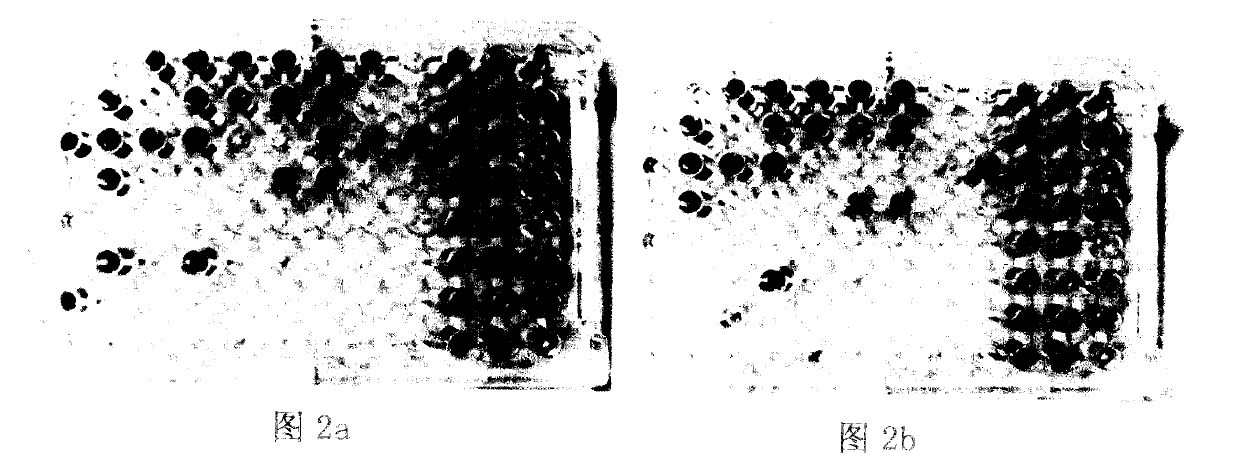
[0020] Embodiment 2, the biolog test of Enterococcus faecium LCT-EF301
[0021] 1) Bacterial suspension preparation: Take out the slant surfaces of the space strain Enterococcus faecium LCT-E F301 and the ground control strain, dip the bacteria with a sterilized bamboo stick and inoculate them in LB liquid medium (4ml system) for 24 hours. Then take 2ml of bacterial liquid at 6000r / s, centrifuge for 5min, discard the supernatant medium, and leave the centrifuged bacterial cells. Wash the cells with sterilized normal saline, centrifuge at 6000r / s for 5min, discard the supernatant again, suspend the cells with Biolog inoculum, and finally transfer to 15ml inoculum to adjust the concentration to about 107-108.
[0022] 2) Inoculation: Pour the above bacterial suspension into the inoculation dish, oscillate evenly, add the sample with a 12-channel (or 8-channel) row gun, add 100ul to each Biolog plate hole, a total of 96 wells, after adding the sample, cover Cover the Biolog plat...
Embodiment 3
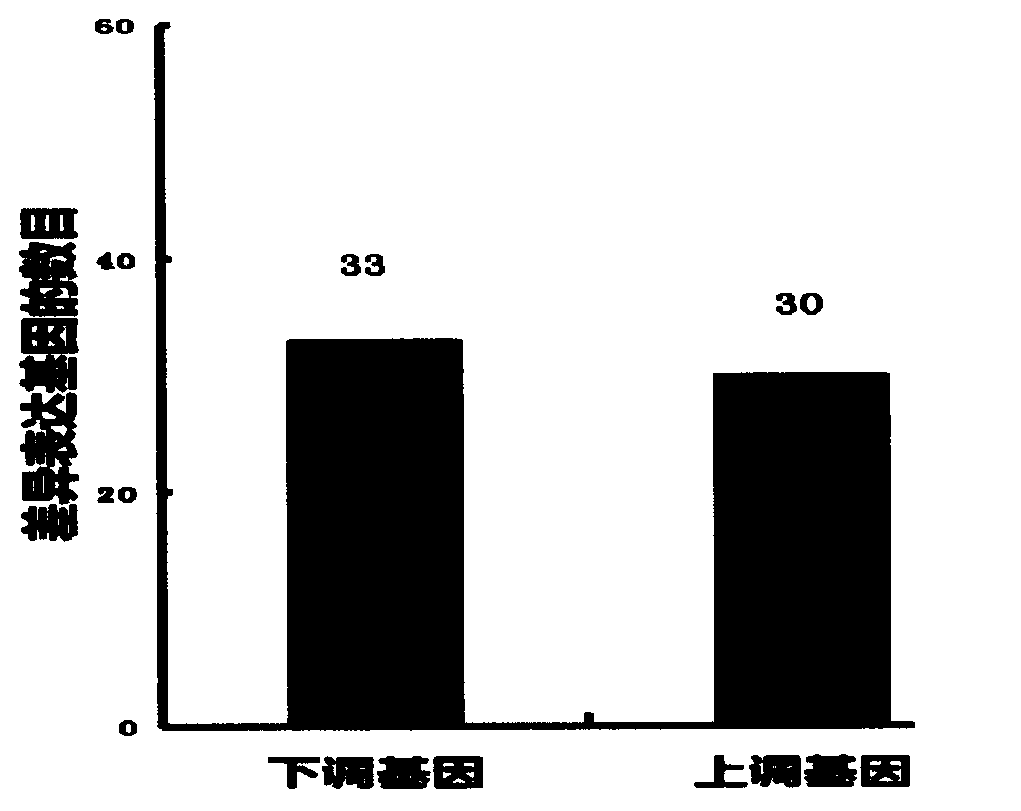
[0025] Example 3. Genome, transcriptome sequencing and proteomic analysis of Enterococcus faecium LCT-EF301
[0026] The whole genome sequence of Enterococcus faecium LCT-EF301 was performed using high-throughput Illumina sequencing technology. The whole genome sequence of Enterococcus faecium LCT-EF301 includes 14 Scaffolds. The whole genome sequence is 2,696,027bp long and has 1 SNP site. type, with a GC content of 37.98%, and 2,639 potential protein-coding genes.
[0027] After extracting the total RNA of Enterococcus faecium LCT-EF301 strain, use the kit to remove the rRNA, add fragmentation buffer to break the mRNA into short fragments, and use the mRNA as a template to synthesize the first cDNA strand with six base random primers (random hexamers) , then add buffer, dNTPs, RNase H and DNA polymerase l to synthesize the second cDNA strand, after purification by QiaQuick PCR kit and elution with EB buffer, do end repair, add polyA and connect the sequencing adapter, and th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com