Method for converting polycyclic aromatic hydrocarbons into monocyclic aromatic hydrocarbons
A technology for single-ring aromatics and polycyclic aromatics, applied in the field of conversion of polycyclic aromatics to single-ring aromatics, can solve the problems of low selectivity, low yield of single-ring aromatics, low conversion depth of polycyclic aromatics, etc. High speed, good technical effect, and the effect of delaying the inactivation rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
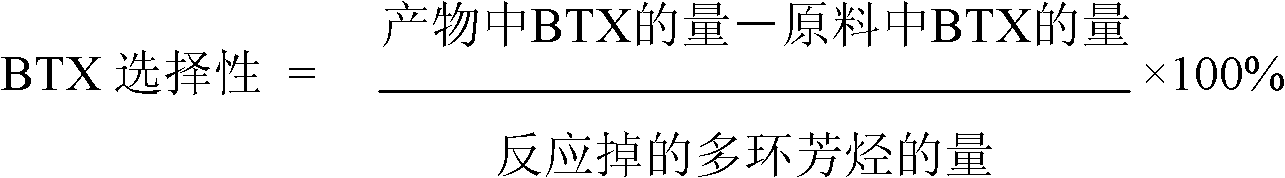
Image
Examples
Embodiment 1
[0030] The dry basis is 30g FAU type zeolite (commercially available hydrogen type), 10g BEA type zeolite (commercially available hydrogen type) and 60g γ-Al 2 o 3 (Industrial product) Add to the mixer until uniform, then add 3g of scallop powder, 5g of HNO at a volume ratio of 1:1 to the mixture 3 solution and 40g deionized water, and grind evenly to make a dough suitable for extrusion. It is extruded through a die, and the shape is a slender cylinder (diameter 1.7mm). After drying at 120°C, it is baked at 550°C for 4h, and then it is cut into carrier particles (1.7×4.0mm) with the same size, recorded as Z1.
[0031] Make a solution of chloroplatinic acid and impregnate the carrier particles at 40°C. The amount of Pt contained in the impregnating solution is 0.1% of the weight of the dry basis of the carrier. After 8h of impregnation and drying, it is calcined at 450°C for 3h to obtain a finished catalyst. a.
Embodiment 2~6
[0033] According to the preparation method and process provided in Example 1, a series of finished catalysts were prepared by changing the types of metal precursors in the impregnation solution during the impregnation of Z1 carrier particles, as shown in Table 1.
[0034] Table 1
[0035] Catalyst number
[0036] F
Embodiment 7~12
[0038] According to the preparation method and process provided in Example 1, a series of finished catalysts were prepared by changing the amount of metal impregnated during the impregnation of Z1 carrier particles, as shown in Table 2.
[0039] Table 2
[0040] Catalyst number
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com