Method and agent for detecting drugs in beverages
A technology of medicaments and medicines, applied in the field of medicines and reagents used to detect beverages
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
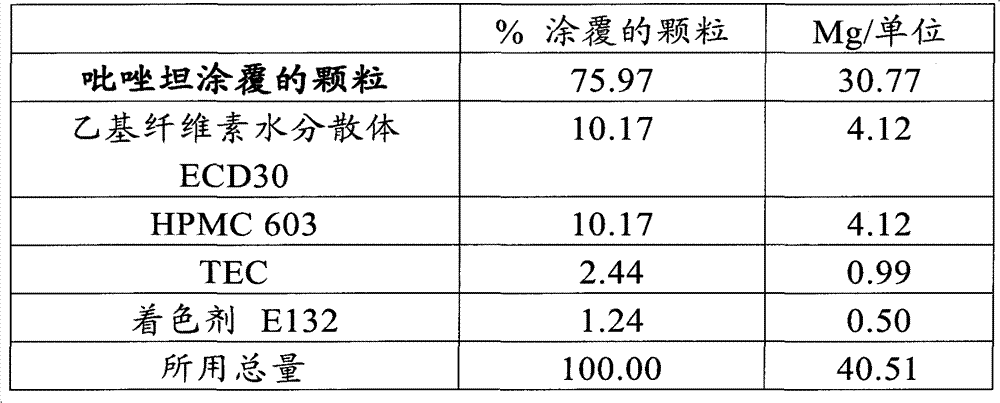
[0138] Orodispersible tablets containing 5 mg zolpidem and having the following composition were prepared:
[0139] components
%
mg / unit
Zolpidem Granules
32.8
41.05
10.0
12.50
43.7
54.57
Crospovidone
10.0
12.50
Colorant E132
0.4
0.50
1.0
1.25
spices
0.1
0.13
silica
1.0
1.25
1.0
1.25
total
100.0
125.0
[0140] Orodispersible tablets are prepared as follows.
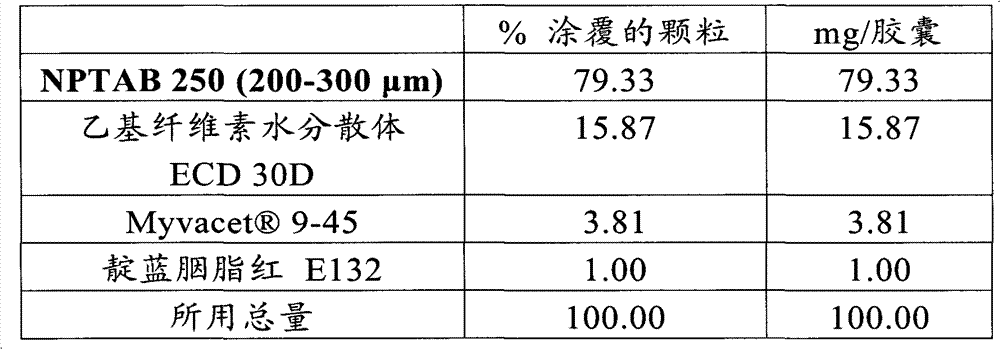
[0141] Zolpidem granules are first prepared with the following percentage composition:
[0142] NPTAB 190 (180-220μm)
56
13
Hypromellose 603
7
1N HCl
2
Ethyl cellulose aqueous dispersion ECD30
13
Hypromellose 603
6
3
...
Embodiment 2
[0153] A conventional immediate release tablet containing 10 mg zolpidem was prepared.
[0154]
%
Mg / unit
Zolpidem Granules
32.8
82.0
10.0
25.0
32.7
81.75
20.0
50.0
povidone
3.0
7.5
silica
0.9
2.25
Colorant E132
0.1
0.25
0.5
1.25
total
100.0
250.0
[0155] Pizotan granules are first prepared. Pizotan granules were prepared as in Example 1.
[0156] The pyrazolidine granules were then mixed with the excipients described in the table above. The powdered mixture is then compressed into tablets.
[0157] Then dissolve one tablet in a glass of pulpless orange juice. Immediately after adding the agent and stirring, a green color and cloudiness develop in a manner visible to the naked eye.
Embodiment 3
[0159] Two types of orodispersible tablets each containing 10 mg of pyrzotan and having the following formulations were prepared:
[0160] components
%
mg / unit
Zolpidem Granules
32.8
82.1
9.6
23.90
30.0
75.00
Crospovidone
5.0
12.50
floating particles
20.0
50.00
Colorant E132
1.0
2.50
spices
0.1
0.25
silica
1.0
2.50
0.5
1.25
total
100.0
250.0
[0161] These tablets were prepared as in Example 1 using the ingredients in the above table.
[0162] For the first series of tablets (C1), the levitating granules were prepared as follows:
[0163] NPTAB 190 (180-220 [mu]m) blanks were coated with an aqueous dispersion of ethylcellulose, triacetin and talc. The coating factor was 30% dry mass and the talc / polymer ratio...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| hardness | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com