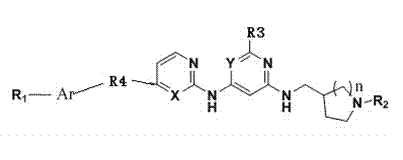
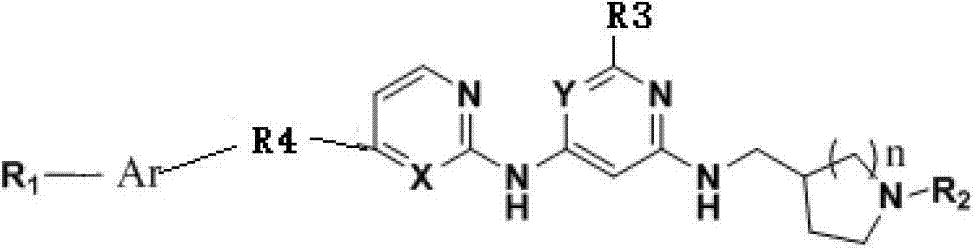
Bcr/Abl tyrosine kinase inhibitor as well as preparation method and application thereof in treating chronic granulocytic leukemia
A technology of tyrosine kinase and chronic granulocytes, applied in the field of Bcr/Abl tyrosine kinase inhibitors, can solve problems such as ineffectiveness, and achieve good inhibitory effect, drug safety, and convenient clinical drug use
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Example 1 (E)-N2-(cyclopropylmethyl)-N4-((1-methyl-tetrahydropyrrolyl)-3-methyl)-N6-(4-(2-(2-naphthaleneethylene base)-2-pyrimidinyl-2,4,6-triaminopyrimidine (VIax1)
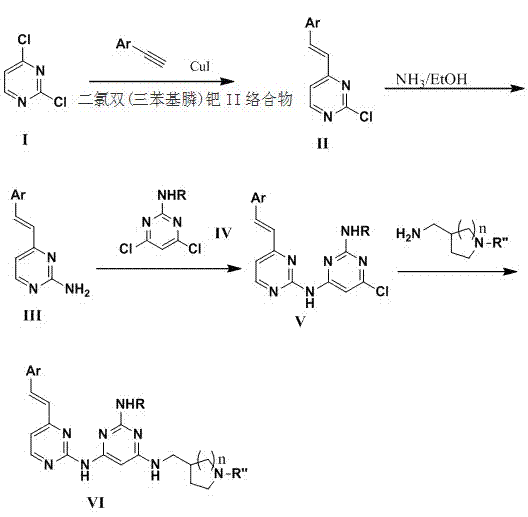
[0040]1. Add 2,4-dichloropyrimidine (14.5g, 97.5mmol), dichlorobis(triphenylphosphine) palladium (II) complex (1.3g, 2.6mmol) into a 500ml round bottom flask, iodide Cuprous (25mg, 0.13mmol), tetrahydrofuran (250ml), triethylamine (34ml, 263mmol). After the mixture was heated to 50°C, a solution of naphthaleneacetylene (97.5 mmol) in tetrahydrofuran (100 ml) was added. After 5 hours, the reaction solution was cooled and diluted with dichloromethane. The organic phase was separated, washed with water and saturated brine, and dried over anhydrous magnesium sulfate. The concentrated crude product was purified by column chromatography to obtain 18.5 g of product intermediate IIa (R1=2-naphthyl).
[0041] 2. Add IIa (16.0g) and saturated ammonia ethanol solution (200ml) into a tube. The system was heated w...
Embodiment 2
[0048] Example 2 (E)-N2-(cyclopropylmethyl)-N4-((1-phenyl-tetrahydropyrrolyl)-3-methyl)-N6-(4-(2-(2-naphthaleneethylene base)-2-pyrimidinyl-2,4,6-triaminopyrimidine (VIax2)
[0049] In a 10ml round bottom flask, add the intermediate Vax (100mg), solvent (3ml) and (1-phenyl-tetrahydropyrrolyl)methylamine (3 equivalents) prepared by the method in Example 1, and the reaction mixture is heated and stirred for 18 Hours, after cooling, add water to dilute and precipitate out. After filtration and purification, 59.85 mg of the final product compound VIax2 (R1=2-naphthyl, R3=cyclopropylmethyl, R2=phenyl, n=1) was obtained. Its structural formula is as follows.
[0050]
[0051] The obtained compound spectral data is:
[0052] LCMS m / z=569 (M+H); 1H-NMR (DMSO-d6, 400MHz): δ8.30 (d, 1H), 7.30-8.15 (m, 7H), 6.80-7.30 (d, 7H).
Embodiment 3
[0053] Example 3 (E)-N2-(cyclopropylmethyl)-N4-tetrahydropyrrolyl-3-methyl)-N6-(4-(2-(2-naphthylvinyl)-2-pyrimidinyl- Preparation of 2,4,6-triaminopyrimidine (VIax3)
[0054] In a 10ml round bottom flask, add the intermediate Vax (100mg) prepared by the method in Example 1, solvent (3ml), (1-Boc-tetrahydropyrrolyl)methylamine (3 equivalents), and the reaction mixture was heated and stirred for 18 hours After cooling, add water to dilute and precipitate out. After filtration and drying, the product was dissolved in 25% TFA in dichloromethane solution, concentrated after 4 hours of reaction, and the crude product was purified to obtain the final product compound VIax3 (R1=2-naphthyl, R3=cyclopropylmethyl, R2=H, n=1) 32.55 mg. Its structural formula is as follows
[0055] .
[0056] The obtained compound spectral data is:
[0057] LCMS m / z=493(M+H); 1H-NMR(DMSO-d6,400MHz):δ8.30(d,1H),7.30-8.05(m,7H),7.10(d,1H),7.01( d,1H),6.85(d,1H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com