Diphenyl ether compounds used in the treatment of liver disease, lung disease, diabetic complications and cardiovascular disease
A compound and liver disease technology, applied in the direction of cardiovascular system diseases, urinary system diseases, diseases, etc., can solve the problems of fat production rate increase, rate decrease, fatty acid β-oxidation rate decrease, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment -1
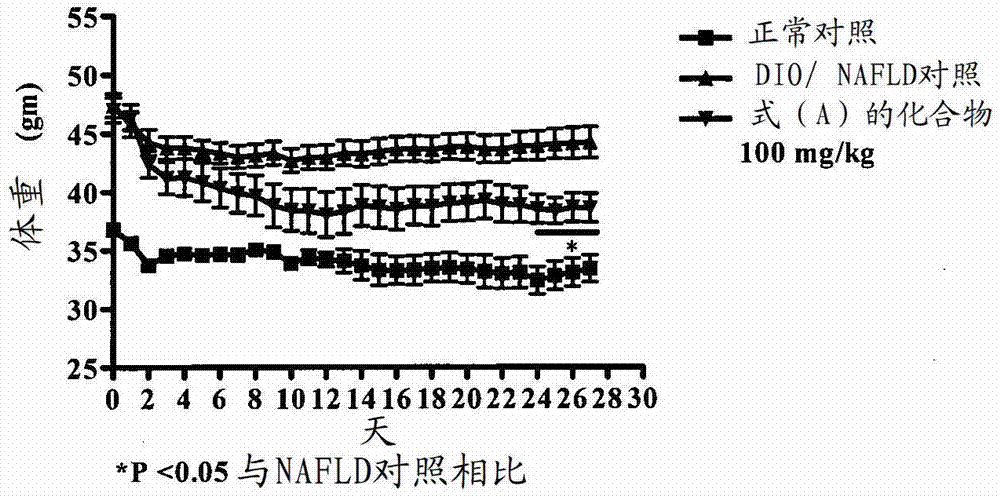
[0160] Embodiment-1: the effect of the compound of formula (A) on the body weight of DIO / NAFLD mice
[0161] Rodent obesity and NAFLD models were established by feeding a supranutritional diet capable of stimulating multiple features of human obesity and NAFLD. These features are processed as follows. Thirty C57BL / 6 male mice, 6-8 weeks old, weighing 16-22 g, were used for this study. Animals were divided into 2 groups. The first group (n=8, where n refers to the number of animals) was fed with chow feed (Nutri Rodent, Tetragon Chemie Pvt.Ltd., Bangalore) were fed as a normal control, and the second group (n=20) was fed with a 60Kcal% high-fat diet (HFD) (diet used for research, New Brunswick, NJ, Cat#D12492 with blue dye) for 90 days. Animals were selected based on their body weight. Animals fed with normal diet (n=8) served as normal control (Group I), and animals fed with HFD were divided into two groups: DIO / NAFLD control (Group II) (n=10) and those treated with 100 ...
Embodiment -2
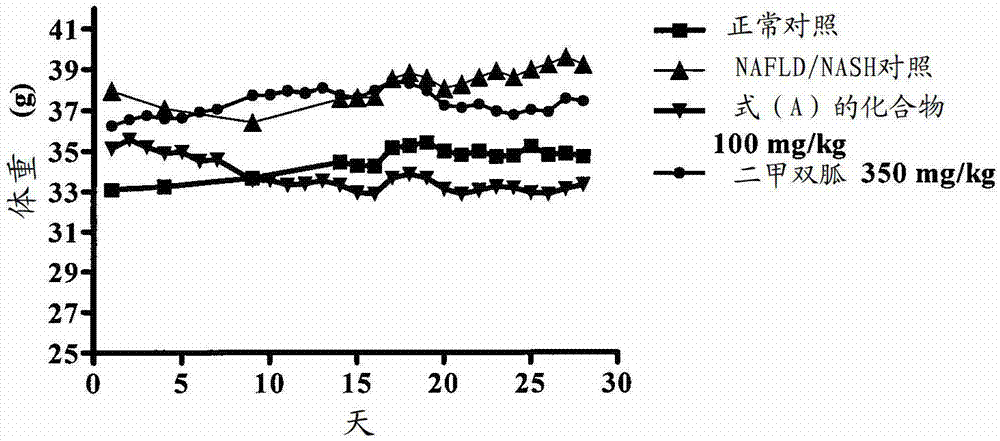
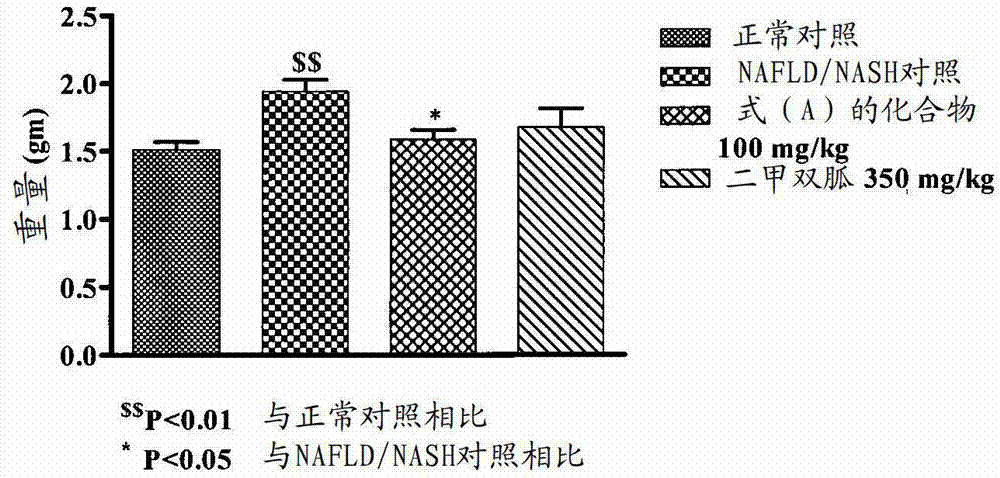
[0162] Example-2: Effect of Compound of Formula (A) on NAFLD / NASH Mice
[0163] A rodent model of NAFLD / NASH was established by feeding a supranutrient diet capable of stimulating multiple features of NAFLD / NASH. These features are processed as follows. Fifty C57BL / 6 male mice, 6-8 weeks old, weighing 16-36 g, were used for this study. Animals were divided into 2 groups. The first group (n=10) was fed with feed (Nutri Rodent, Tetragon Chemie Pvt.Ltd., Bangalore) were fed as a normal control, and the second group (n=40) was fed with 60Kcal% HFD (diet used for research, New Brunswick, NJ, Cat#D12492 with blue dye) and 40 % high fructose liquid (HFL) feeding for 60 days. After 45 days, two animals from the disease induction and normal control groups were sacrificed for liver histopathological examination to confirm the induction of NAFLD / NASH. Animals fed with normal diet served as normal control (group I), animals fed with HFD and HFL were divided into 3 groups (n=6-8): NA...
Embodiment -3
[0164] Example-3: Effect of Compound of Formula (A) on NAFLD / NASH Mice
[0165] According to the scheme in Example-2. The compound of formula (A) was compared with other antidiabetic compounds (Figure-8-13).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com