Universal method for enriching glycopeptides by metallic oxide
A general method and oxide technology, applied in the field of biochemical analysis, can solve the problems of insufficient versatility and unsuitability of the method, and achieve the effects of high glycosylation coverage, easy repeatability, and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
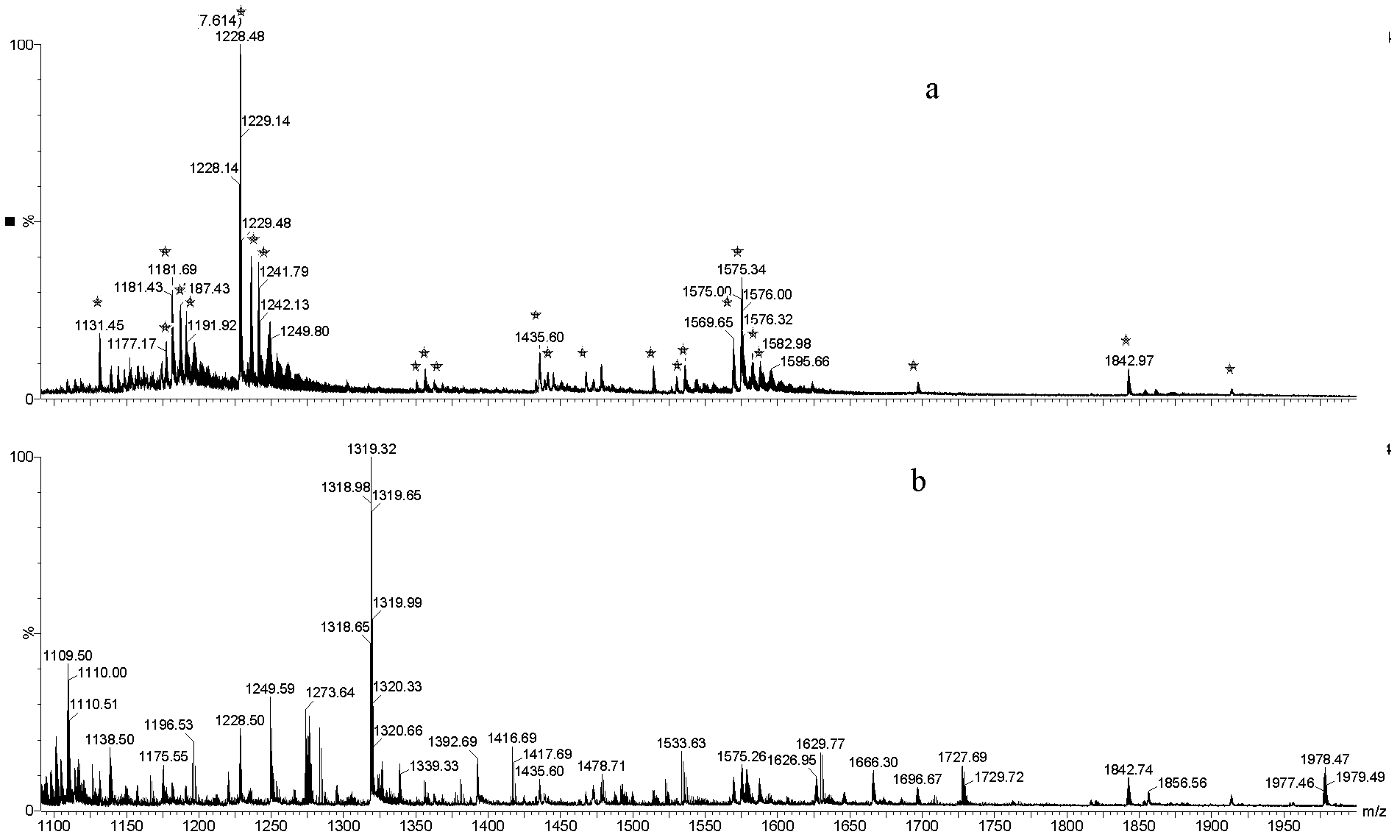
[0024] Put 1 mg of titanium oxide into a gel tip, load 10 μL of transferrin hydrolyzate (see Preparation of sample solution) (pH 3), and then elute twice with 30 μL of 0.25% ammonia solution (pH 3). 10); finally, eluted twice with 30 μL of 10% ammonia solution (PH=12) by volume to obtain the glycopeptide. The enriched glycopeptides were analyzed by mass spectrometry.
[0025] Depend on figure 1 It can be seen that the glycopeptides in the hydrolyzate of transferrin can be specifically enriched and purified by titanium oxide.
Embodiment 2
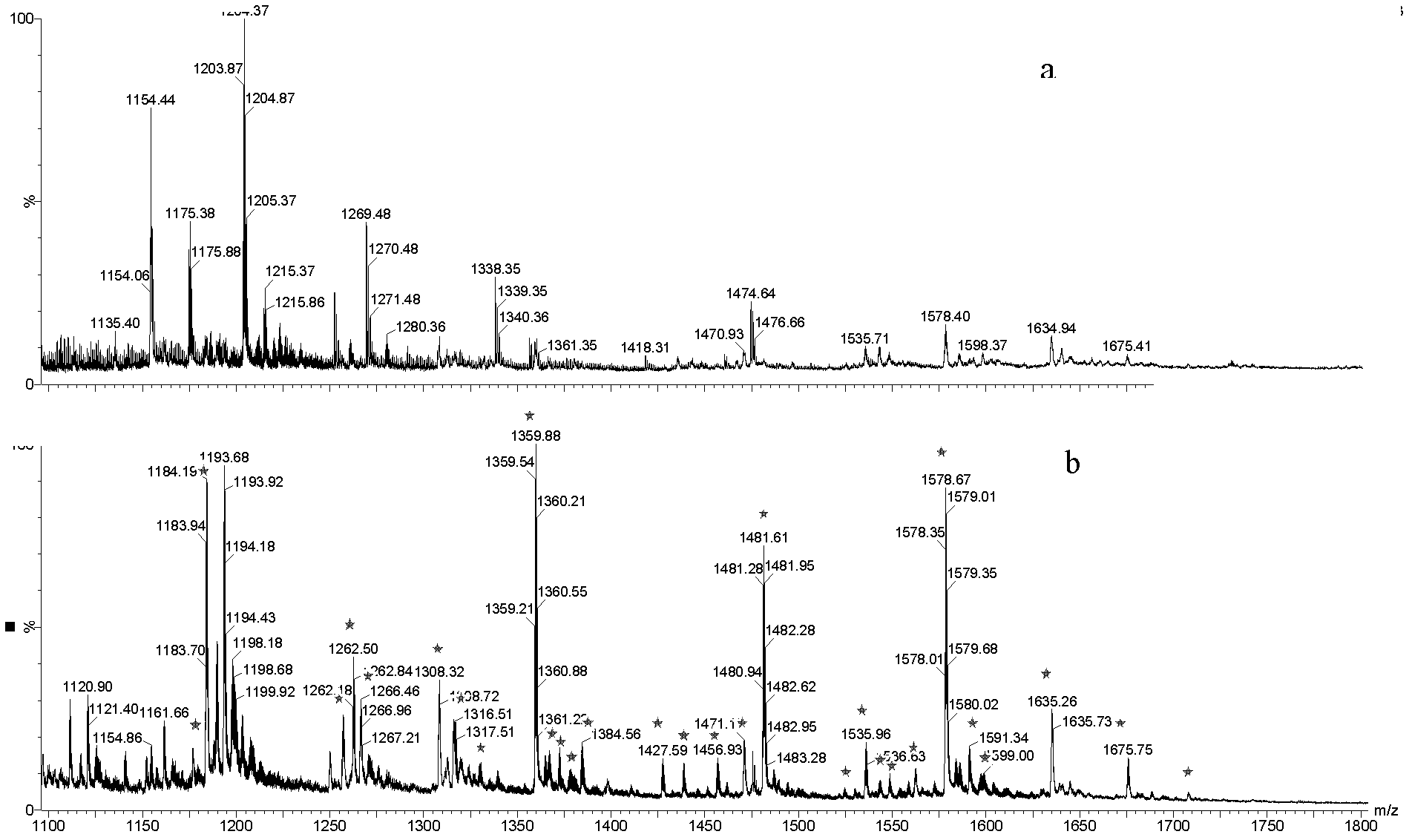
[0027] Adjust the operation mode of enrichment to centrifugation, put 1 mg of titanium oxide into a centrifuge tube, mix with 5 μL of peptiglobulin hydrolyzate (pH 3), incubate for 5 minutes, collect the precipitate after centrifugation; then use 30 μL of the volume concentration of the precipitate to be 10% CH 3 CN / 0.1%NH 4 OH (pH 10) was incubated for 5 min, and the precipitate was collected. Incubate the precipitate with 30 μL of 0.25% ammonia solution (PH=10) for 5 min, collect the precipitate after centrifugation, repeat the incubation and centrifugation steps twice; finally incubate the precipitate with 30 μL of 10% ammonia solution (PH=12) After centrifugation for 5 minutes, the supernatant was collected to obtain glycopeptides. Each supernatant was analyzed directly on a MALDI-TOF mass spectrometer.
[0028] Depend on figure 2 It can be seen that the glycopeptides in the fetuin hydrolyzate are enriched and purified by titanium oxide anisotropy.
Embodiment 3-6
[0030] Adjust the weight of the metal oxide material to 2 mg, 3 mg, 6 mg, and 10 mg. Other conditions are the same as in Example 1. The glycopeptide obtained after enrichment is subjected to mass spectrometry analysis. The experimental results show that 1 mg of the material can be effectively retained in the extraction mode operation mode. and enriched glycopeptides in glycoprotein standard digests.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com