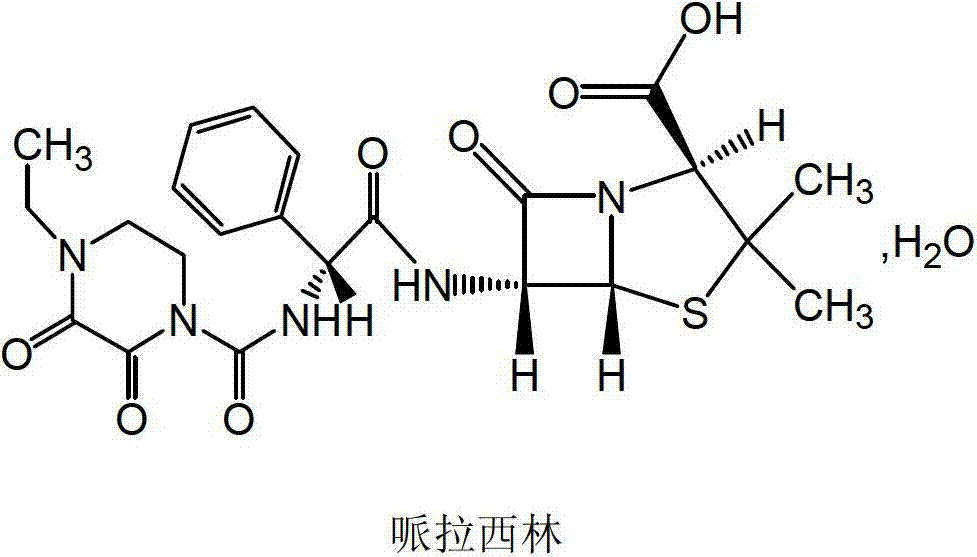
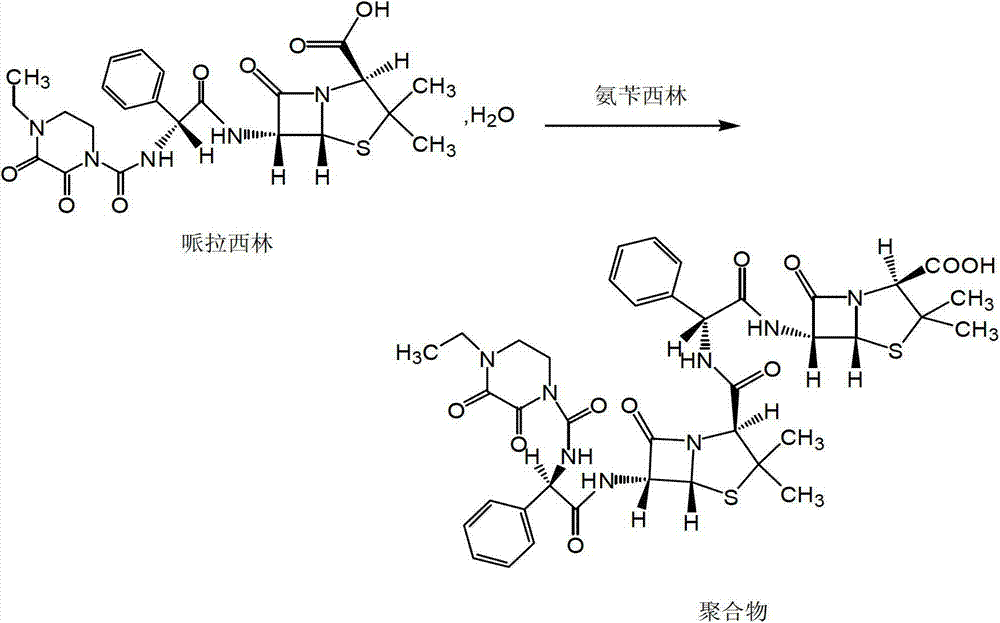
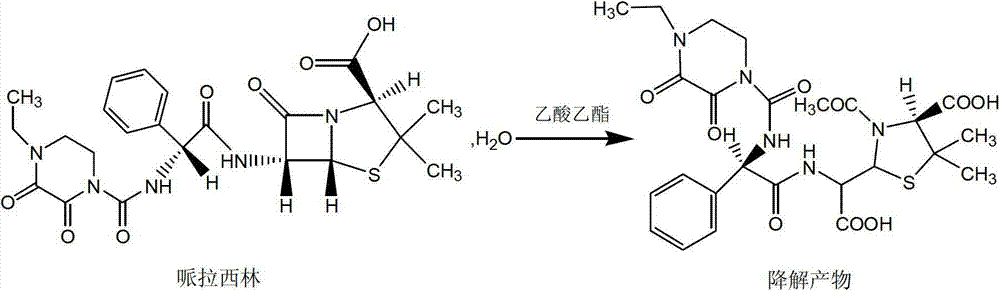
Crystallization method of piperacillin
A piperacillin and piperacillin sodium technology, which is applied in the crystallization field of piperacillin, can solve the problems of high solvent residue and difficult polymer impurities, and achieve excellent stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Put 200g of piperacillin and 600mL of purified water into a 1000mL three-necked bottle in turn, control the temperature at 5~10°C, slowly add 31.5g of solid sodium bicarbonate, and continue to stir for 60 minutes after the addition is complete until the system is dissolved and clarified. At this time, the pH of the system is 4.95 . Add 1.0g of EDTA-2Na and 10g of activated carbon, stir for 30min to decolorize, and then filter with suction. Transfer the filtrate to a clean three-necked flask, adjust the system temperature to 40~45°C, slowly add 2mol / L hydrochloric acid dropwise until the pH of the system is 4.0, stir and crystallize for 60min. Continue to slowly add hydrochloric acid dropwise until the pH value of the system is 1.0, and stir and crystallize for 60 minutes. Suction filter the reaction system, beat and wash the material with 100 mL of purified water. After drying, 196.5 g of finished white crystalline piperacillin was obtained, with a crystallization yie...
Embodiment 2
[0021] Put 200g of piperacillin and 1200mL of purified water into a 1000mL three-necked bottle in sequence, control the temperature at 5~10°C, slowly add 31.5g of solid sodium bicarbonate, and continue stirring for 60 minutes after the addition is complete until the system is dissolved and clarified. At this time, the pH of the system is 5.05 . Add 1.0g of EDTA-2Na and 10g of activated carbon, stir for 30min to decolorize, and then filter with suction. Transfer the filtrate to a clean three-necked flask, adjust the temperature of the system to 40-45°C, slowly add 2mol / L hydrochloric acid dropwise until the pH of the system is 4.15, stir and crystallize for 60min. Continue to slowly add hydrochloric acid dropwise until the pH value of the system is 1.05, and stir and crystallize for 60 minutes. Suction filter the reaction system, beat and wash the material with 100 mL of purified water. After drying, 192.3 g of finished white crystalline piperacillin was obtained, with a crys...
Embodiment 3
[0023] Put 200g of piperacillin and 1800mL of purified water into a 1000mL three-neck bottle in turn, control the temperature at 5~10°C, slowly add 31.5g of solid sodium bicarbonate, and continue stirring for 60 minutes after the addition is complete until the system is dissolved and clarified. At this time, the pH of the system is 5.10 . Add 1.0g of EDTA-2Na and 10g of activated carbon, stir for 30min to decolorize, and then filter with suction. Transfer the filtrate to a clean three-necked flask, adjust the temperature of the system to 40~45°C, slowly add 2mol / L hydrochloric acid dropwise until the pH of the system is 4.10, stir and crystallize for 60min. Continue to slowly add hydrochloric acid dropwise until the pH value of the system is 1.13, and stir and crystallize for 60 minutes. Suction filter the reaction system, beat and wash the material with 100 mL of purified water. After drying, 192.8 g of finished white crystalline piperacillin was obtained, with a crystalliz...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com