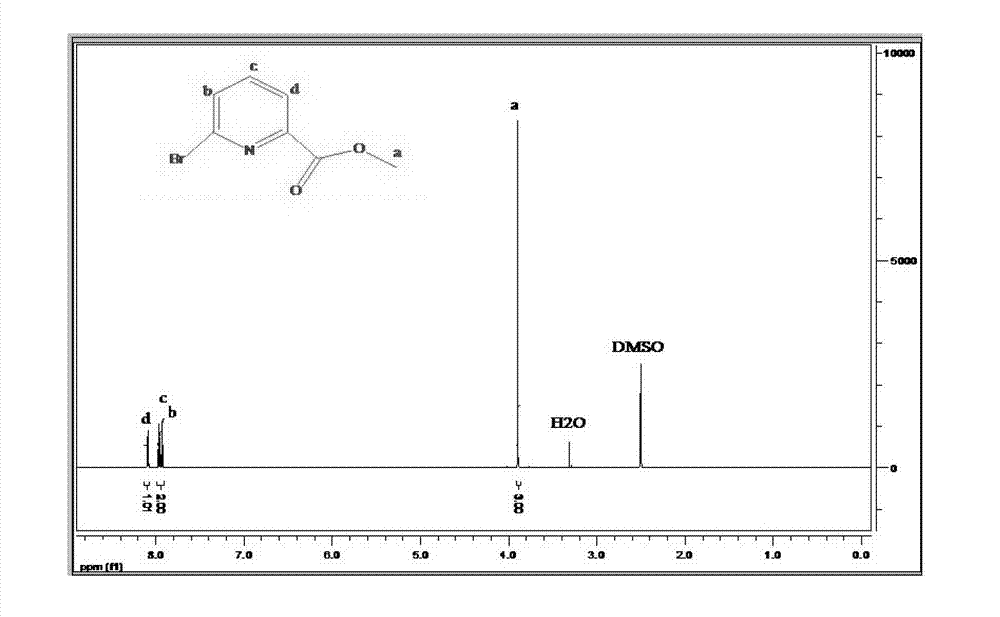
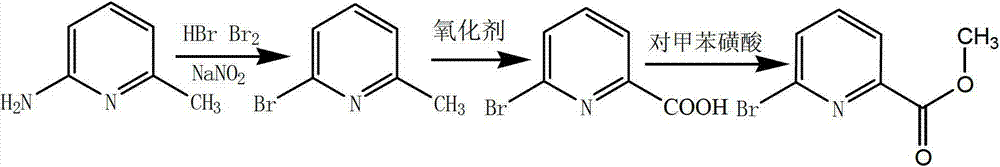
Preparation method of 6-bromine-2-pyridine methyl formate
A technology of methyl picolinate and picolinic acid, which is applied in the field of preparation of methyl 6-bromo-2-picolinate, can solve the problems of not being suitable for large-scale industrial production, insufficient product purity, troublesome post-processing, etc. The effect of preparation yield, less process pollution and easy separation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0025] The preparation method of 6-bromo-2-picolinic acid methyl ester provided by the invention comprises:
[0026] Add anhydrous methanol, 6-bromo-2-pyridinecarboxylic acid and p-toluenesulfonic acid into the reaction vessel, wherein the molar ratio of 6-bromo-2-pyridinecarboxylic acid to anhydrous methanol is 1:40-60, 6-bromo-2-pyridinecarboxylic acid - The molar ratio of 2-pyridinecarboxylic acid to p-toluenesulfonic acid is 1:0.06-0.2, preferably 1:0.1-0.16, heated to reflux under stirring for 2-8 hours, preferably refluxed for 4-6 hours, stop heating after the reaction is completed, continue Stir to cool to room temperature.
[0027] After the reaction system is spin-dried, the solid is dissolved in organic solvents such as ethyl acetate, dichloromethane or ether, washed with saturated sodium bicarbonate or sodium carbonate solution, and then washed with water for 2-3 times, and dried with a desiccant such as anhydrous Magnesium sulfate, sodium sulfate, calcium sulfate,...
Embodiment 1
[0041] (1) Preparation of 6-bromo-2-methylpyridine
[0042] Add 46mL of hydrobromic acid with a concentration of 48% and 10.8g of analytically pure 2-amino-6-picoline into the reaction flask, slowly add 6.2mL of liquid bromine dropwise at -10°C, complete the dropwise addition for 0.5h, and react for 1.5h; At -10°C-0°C, 22.8 mL of aqueous sodium nitrite solution with a concentration of 25% by mass was added dropwise. After the dropwise addition was completed, the reaction was carried out at 15°C for 0.5 h; Reacted for 1 h, left to stand and separated, the aqueous phase was extracted 3 times with 200 mL of dichloromethane, the organic phases were combined, washed with water, the organic phase was concentrated, and distilled under reduced pressure to obtain 15.8 g of light yellow liquid with a calculated yield of 92%. The purity of 6-bromo-2-picoline product obtained by phase chromatography is 99.3%.
[0043] (2) Preparation of 6-bromo-2-pyridinecarboxylic acid
[0044] Add 15....
Embodiment 2-5
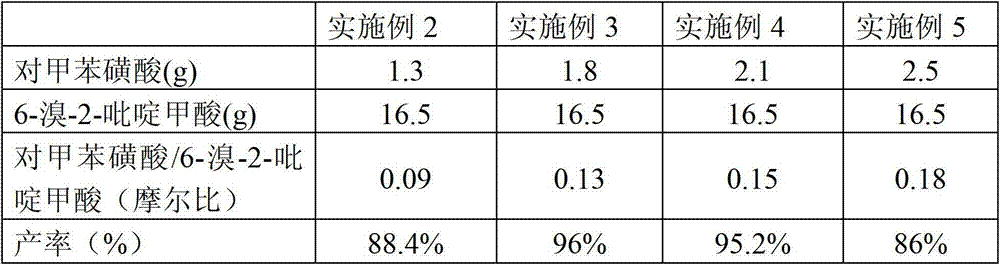
[0048] Embodiment 2-5 differs from Example 1 in that the molar ratio of p-toluenesulfonic acid and 6-bromo-2-pyridinecarboxylic acid is shown in Table 1 below, and other steps and conditions are the same as in Example 1, and the yield obtained They are listed in Table 1 respectively.
[0049] Table 1
[0050]
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com