Water-soluble asymmetric indocyanine fluorescent dye and preparation method thereof
A fluorescent dye, asymmetric technology, used in organic dyes, luminescent materials, chemical instruments and methods, etc., can solve the problems of many side reactions, fluorescence quenching, fluorescence intensity drop, etc., to achieve strong light stability and fluorescence intensity, The effect of high fluorescence quantum yield and reduction of self-aggregation tendency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
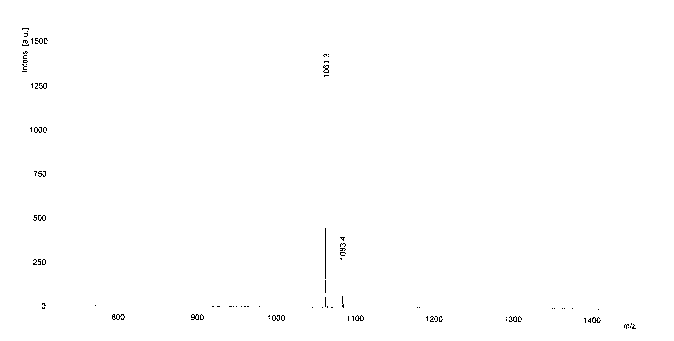
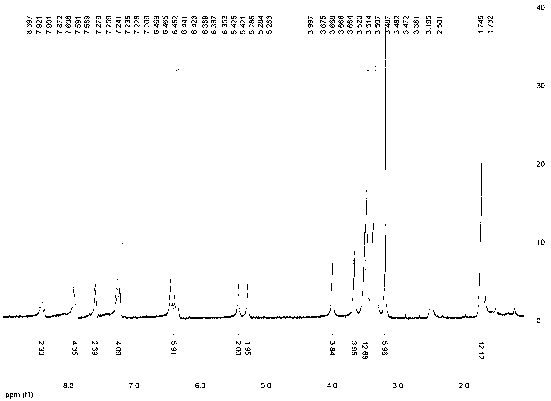
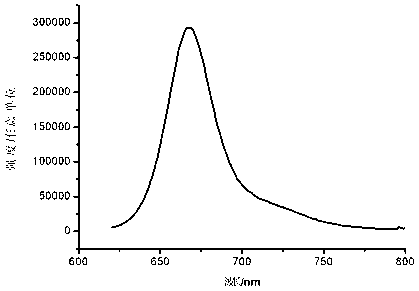
[0033] Water-soluble asymmetric indocyanine fluorescent dye (Cy5-671), its structure is:
[0034]
[0035] The following are the synthetic route diagram and specific preparation method of the compound:
[0036] (1) Synthetic roadmap
[0037]
[0038]
[0039] (2) Preparation method
[0040] (1) Synthesis of intermediate A2,3,3-trimethyl-5-sulfonic acid-3H-indole
[0041] Add 20mL of acetic acid, 5.0g (0.026mol) of 4-sulfophenylhydrazine and 6.0mL (0.052mol) of 3-methyl-2-butanone into a 50mL three-necked flask in sequence, and react at 80°C under argon protection for 2.0 h, acetic acid was distilled off under reduced pressure, dissolved in 20 mL of methanol and then added with 100 mL of acetone to precipitate a pink solid, namely intermediate A, with a yield of about 57%.
[0042] (2) Synthesis of Intermediate B N-p-carboxybenzyl-2,3,3-trimethyl-5-sulfonic acid-3H-indole
[0043] Add 20mL of toluene, 1.0g (0.0042mol) of intermediate A, 0.4g (0.0042mol) of potassium ...
Embodiment 2
[0073] Water-soluble asymmetric indocyanine fluorescent dye (Cy3-567), its structure is:
[0074]
[0075] The preparation method is:
[0076] (1) N-(3,5-bis(2-(2-methoxyethoxy)ethoxy)ethoxy)benzyl-2,3,3-trimethyl-5-sulfonic acid group- Synthesis of 3H-indole
[0077] Add 30mL ethanol, 0.0042mol2,3,3-trimethyl-5-sulfonic acid group-3H-indole, 0.0126mol potassium acetate and 0.0126mol3,5-bis(2-(2- Methoxyethoxy)ethoxy)ethoxybenzyl bromide, reflux reaction under argon protection for 20h, after the reaction, ethanol was rotary evaporated, and the methanol / dichloromethane mixed solvent with a volume ratio of 1:2 was used as the washing The solvent was removed, and the crude product was separated with a silica gel-filled separation column to obtain a red solid product with a yield of 61%.
[0078] (2) Synthesis of the target product
[0079]Add 6.0mL acetic acid, 6.0mL acetic anhydride, 0.0015mol N-p-carboxybenzyl-2,3,3-trimethyl-5-sulfonic acid-3H-indole, 0.0015mol N ,N′-D...
Embodiment 3
[0085] Water-soluble asymmetric indocyanine fluorescent dye Cy7, the structure is:
[0086]
[0087] Its preparation method is as follows:
[0088] (1) N-(3,5-bis(2-(2-methoxyethoxy)ethoxy)ethoxy)benzyl-2,3,3-trimethyl-5-sulfonic acid group- Synthesis of 3H-indole
[0089] Add 30mL methanol, 0.0042mol2,3,3-trimethyl-5-sulfonic acid group-3H-indole, 0.0042mol potassium acetate and 0.0042mol3,5-bis(2-(2- Methoxyethoxy) ethoxy) ethoxybenzyl bromide, reflux reaction under the protection of argon for 40h, after the reaction, methanol was rotary evaporated, and the methanol / dichloromethane mixed solvent with a volume ratio of 1:2 was used as the washing The solvent was removed, and the crude product was separated with a silica gel-filled separation column to obtain a red solid product with a yield of 61%.
[0090] (2) Synthesis of the target product
[0091] Add 6.0mL acetic acid, 6.0mL acetic anhydride, 0.0015mol N-p-carboxybenzyl-2,3,3-trimethyl-5-sulfonic acid-3H-indole, 0...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Maximum emission wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com