Synthesis process of candesartan cilexetil
A technology of candesartan medoxomil and a synthesis process, which is applied in the field of synthesis technology of candesartan medoxomil, can solve the problem that the treatment of tin-containing wastewater is difficult, increases the treatment cost of "three wastes", and increases the technological process of candesartan medoxomil. Difficulty in production and other problems, to achieve the effect of cheap price, simple processing, and improved reaction yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
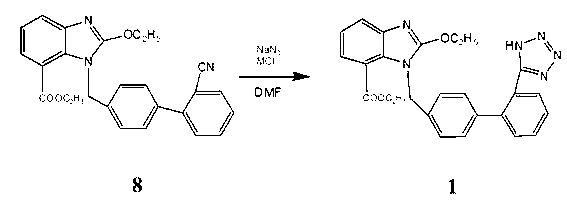
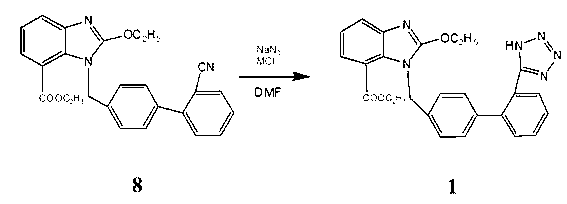
[0017] 8.22g (0.02mol) intermediate 8( 1-[(2'-cyano-1,1'-biphenyl-4-yl)methyl]-2-ethoxy-7-benzimidazolecarboxylic acid ethyl ester) dissolved in 30mL DMF (0.388 mol), add 1.43g (0.022mol) NaN 3 and 1.18g (0.022mol) NH 4 Cl, heat up to 135-140°C for 24 hours, cool down to room temperature, distill off DMF under reduced pressure, add 100mL of water, wash twice with toluene 50mLx2, then adjust the pH to 6 with 1N HCl, solids precipitate out, filter, and use for filter cake Methanol was recrystallized to obtain 5.7 g of a white solid with a melting point of 190-192°C (literature value: 192-194°C), and a yield of 63.5%. The reaction equation involved in the present invention is:
[0018]
Embodiment 2
[0020] 8.22g (0.02mol) ethyl 1-[(2'-cyano-1,1'-biphenyl-4-yl)methyl]-2-ethoxy-7-benzimidazolecarboxylate Dissolve in 40mL DMF (0.517mol), add 1.56g (0.024mol) NaN 3 and 6.52g (0.048mol) ZnCl 2 , heated up to 160°C for 30 hours, lowered to room temperature, distilled off DMF under reduced pressure, added 100mL of water, washed twice with toluene 50mLx2, then adjusted the pH to 7 with 1N HCl, solid precipitated, filtered, and the filter cake was recrystallized with methanol 6.1 g of white solid was obtained, its melting point was measured at 189-191°C (literature value: 192-194°C), and the yield was 67.1%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com