Synthetic method of osteoporosis treatment drug 1 alpha-hydroxyitamin D3
A technology for hydroxyvitamins and osteoporosis, applied in drug combinations, bone diseases, organic chemistry, etc., can solve the problems of unavoidable loss of product isomers, cumbersome extraction process, inconvenient handling, etc., so as to save the extraction and cleaning of pyridine steps, save cooling energy consumption, and improve the yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Embodiment 1 (original technical solution)
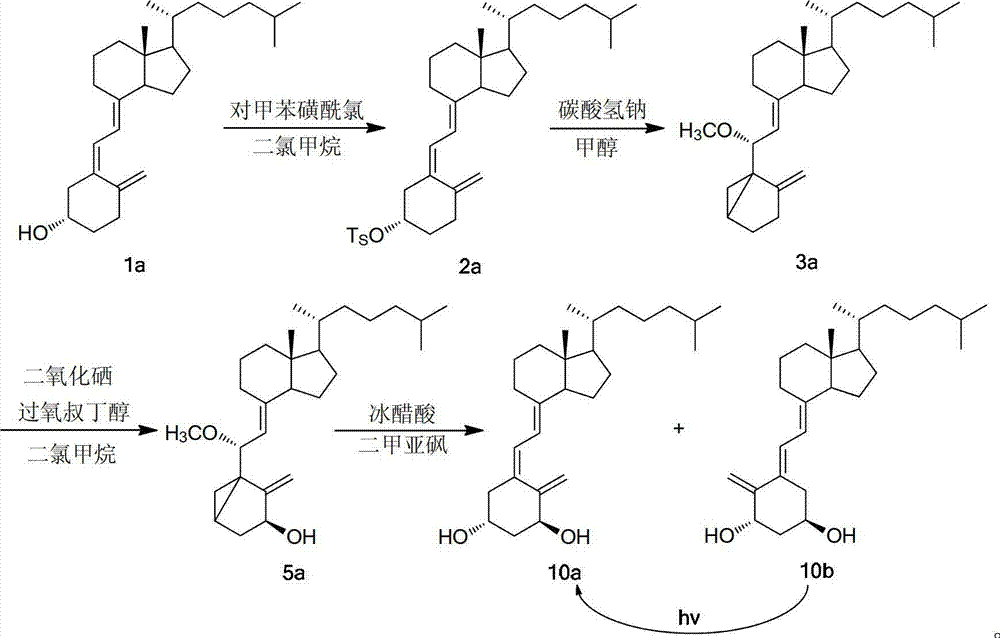
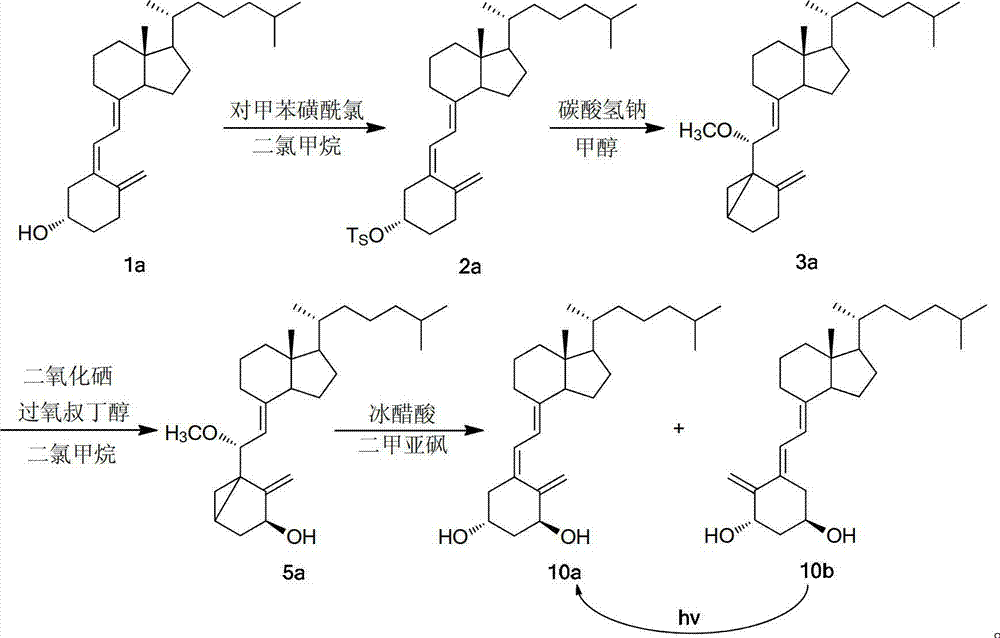
[0035] Weigh 5.00g VD 3 (1a, 0.013mol), put it into a 200mL Erlenmeyer flask, add 30mL of pyridine, put it in an ice-water bath, add 7.14g (0.037mol) of p-toluenesulfonyl chloride into 30mL of pyridine to dissolve, add it to the Erlenmeyer flask several times, Shaking while adding, the color of the system darkened and turned bright yellow. After mixing, it was filled with argon gas for protection, sealed and protected from light, and placed in a refrigerator (at a temperature of about 4°C) for about 48 hours of reaction. Take out after the completion of TLC monitoring reaction, the solution is deep red, and there are colorless transparent needle-like crystals at the bottom. Transfer the liquid into a 500mL beaker, add a little ice cubes, add 100mL saturated NaHCO several times 3 Aqueous solution, stirring while adding, a large number of bubbles generated, adding ethyl acetate for extraction, then transferred to a 500mL sep...
Embodiment 2
[0037] An osteoporosis treatment drug 1α-hydroxyvitamin D 3 The synthetic method comprises the steps:
[0038] (1) Esterification: Vitamin D 3 (1a) and methanesulfonyl chloride were put into benzene, under the protection of nitrogen, adding pyridine and methylamine, at a temperature of 50 ℃, vitamin D 3 The hydroxyl group was subjected to an esterification reaction. After the reaction was monitored by TLC (4 hours), the solution was taken out, the solution was light red, and there were colorless transparent needle-like crystals at the bottom, which were washed twice with saturated sodium bicarbonate solution, and then washed with saturated aqueous sodium chloride solution. Twice, add anhydrous sodium sulfate to dry, filter, and distill under reduced pressure to obtain vitamin D 3 3-Hydroxyester (2a), yield nearly 100%; Vitamin D 3 , the mol ratio of methanesulfonyl chloride, pyridine and methylamine is 1:1.3:5.0:0.1;
Embodiment 3
[0044] An osteoporosis treatment drug 1α-hydroxyvitamin D 3 The synthetic method comprises the steps:
[0045] (1) Esterification: Vitamin D 3 (1a) Put p-toluenesulfonyl chloride in toluene, under the protection of nitrogen, add pyridine and ethylamine, at 40 ° C, the vitamin D 3 The hydroxyl group was subjected to an esterification reaction. After the reaction was completed (4 hours and 15 minutes) by TLC, the solution was taken out, the solution was light red, and there were colorless transparent needle-like crystals at the bottom, washed twice with saturated sodium bicarbonate solution, and then washed with saturated sodium chloride Wash twice with aqueous solution, add anhydrous sodium sulfate to dry, filter, and distill under reduced pressure to obtain vitamin D 3 3-Hydroxyester (2a), yield nearly 100%; Vitamin D 3 , the mol ratio of p-toluenesulfonyl chloride, pyridine and ethylamine is 1:1.3:10.0:1.0;
[0046] (2) Cyclization: Using methanol as solvent, mix 1 mole o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com