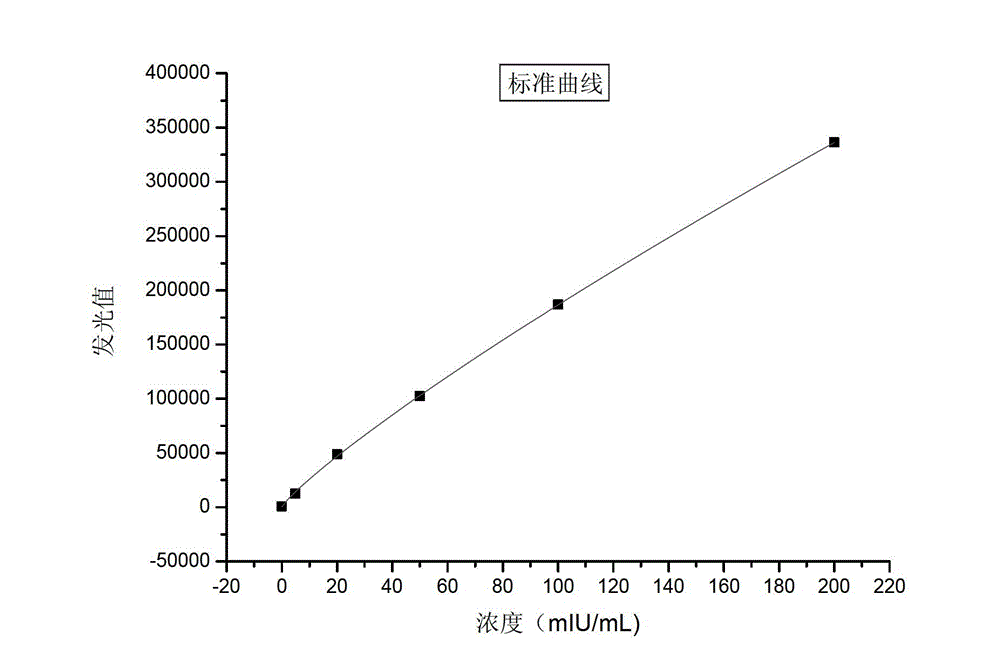
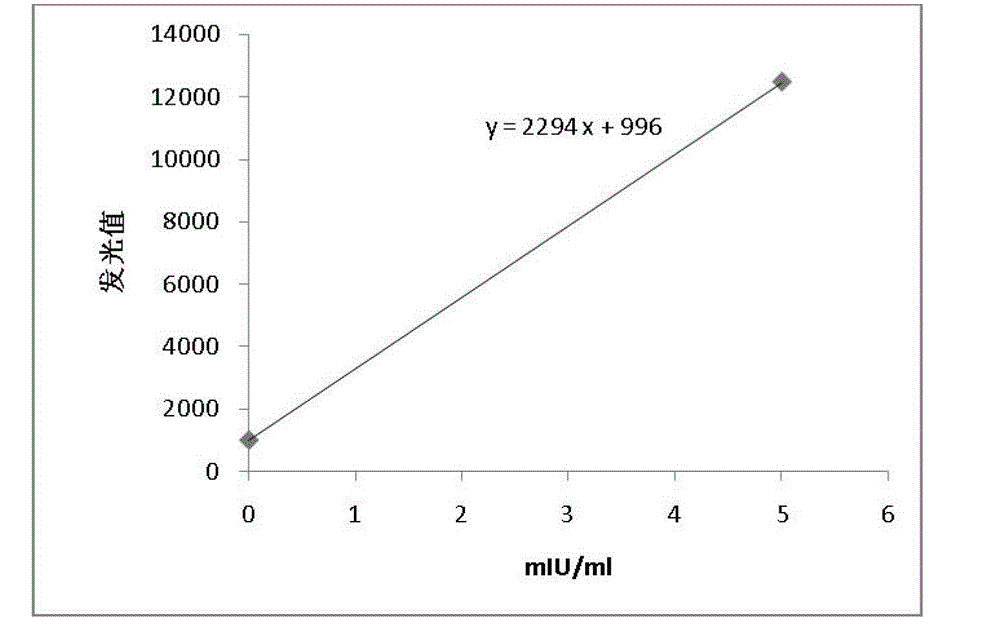
Nanometer magnetic particle chemiluminiscence kit, preparation method and detection method of hepatitis B virus surface-antibody
A hepatitis B virus, nano-magnetic particle technology, applied in chemiluminescence/bioluminescence, biological testing, analysis by chemical reaction of materials, etc., can solve problems affecting detection sensitivity and accuracy, and achieve cost advantages, The effect of high sensitivity and low production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Embodiment 1 The preparation of the first reagent
[0043] (1) Materials and instruments: Hepatitis B virus e antigen monoclonal antibody preserved in phosphate buffer (purity over 95wt%, concentration 2mg / mL); fluorescein isothiocyanate (FITC), sodium carbonate and other reagents It should be chemically pure; G-25 gel purification column was purchased from GE.
[0044] (2) Preparation steps:
[0045] ① Prepare 0.5mg / mL FITC solution with 0.1~0.2mol / L carbonate buffer solution with pH 9.0~10.0;
[0046] ② Add the FITC solution prepared in step ① to the antibody solution according to the ratio of hepatitis B virus e antigen antibody to FITC molecule ratio of 1:20, mix well, let stand at room temperature for 12 hours, and react to generate e antigen antibody-FITC conjugate;
[0047] ③Separate the reaction liquid after step ② through G-25 gel column, remove unreacted FITC, and obtain the antibody-FITC conjugate containing hepatitis B virus e antigen (ie, FITC-labeled he...
Embodiment 2
[0049] Embodiment 2 Preparation of the second reagent
[0050] (1) Materials and instruments: Hepatitis B virus e antigen monoclonal antibody preserved in phosphate buffer (purity over 95wt%, concentration 2mg / mL); alkaline phosphatase preserved in phosphate buffer (ALP solution, The purity of ALP is about 99%, the specific activity is about 1500U / mg, and the concentration is 10mg / mL); the cross-linking agent SMCC, 2-IT are purchased from THERMO company, TRIS and other chemical reagents should be chemically pure; G-25 gel purification Columns are GE products.
[0051] (2) Preparation steps:
[0052] ①Take 1mg of e-antigen antibody, add 2-4μL of 10mg / mL coupling agent 2-IT solution, let stand at room temperature for 20min, add 10μL of 0.1mol / L glycine solution, let stand at room temperature for 5min, and use G-25 gel column Remove the salt, collect the activated CA125 antibody, and store it at 2-8°C for later use;
[0053] ② Take 1.5 mg of ALP solution, add 10-20 μL of 5 mg / ...
Embodiment 3
[0056] The preparation of embodiment 3 magnetic separation reagents
[0057] (1) Materials and instruments:
[0058] Suspension of magnetic particles: the content of magnetic particles is 5wt%, and the magnetic particles contain carboxyl (COOH) active groups. The carboxyl group content per gram (g) of magnetic particles (dry weight) is not less than 0.4 millimoles (mmol), with superparamagnetism, The diameter is between 0.5-2μm.
[0059] Anti-FITC antibody: It can be a polyclonal antibody or a monoclonal antibody, with a purity of more than 90% by weight and a dilution titer of more than 1:1 million;
[0060] 2-Morpholineethanesulfonic acid (MES), carbodiimide (EDC), TRIS, and other reagents should be of chemical purity.
[0061] (2) Preparation steps:
[0062] ①Take 100mg of magnetic particle suspension, magnetically separate to remove the supernatant, and resuspend in 10mL of 0.05mol / L, pH 4.5~5 MES buffer;
[0063] ②Add 2~4mg of anti-FITC antibody and suspend at room te...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| Specific activity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com