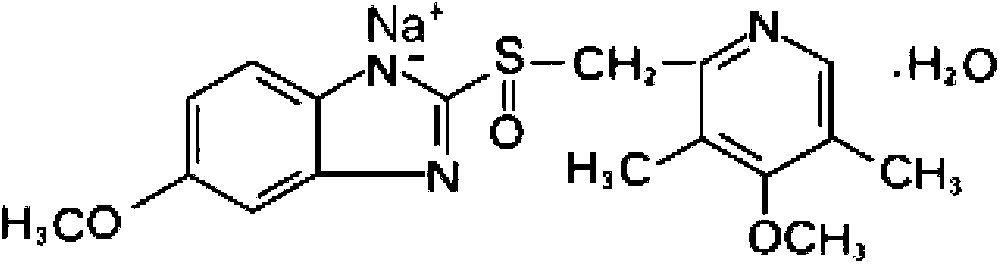
Novel omeprazole compound and pharmaceutical composition thereof
A technology of omeprazole sodium and its compound, which is applied in the field of compounds and pharmaceutical compositions for treating gastric ulcer disease, can solve the problems of poor stability, chemical structure damage, low purity of omeprazole sodium, etc., and achieve improved stability Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
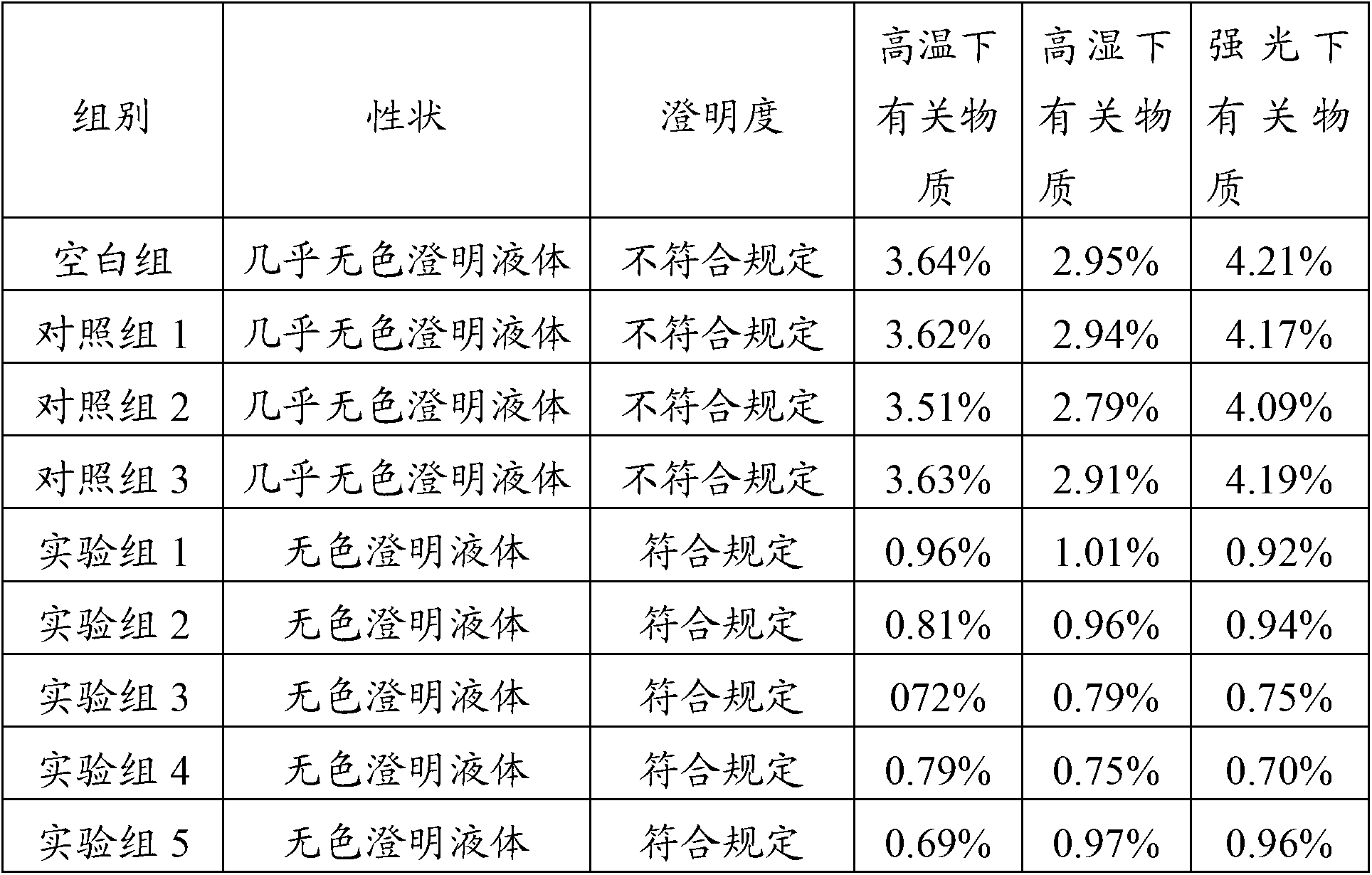
Examples
Embodiment 1
[0018] A kind of pharmaceutical composition of omeprazole sodium compound, described pharmaceutical composition is injection, and described injection is prepared by the following steps:
[0019] (1) Take 400ml of water for injection and place it in a container, then add 250mg of sodium glutamate, 40mg of sodium tartrate and 70mg of vitamin C, dissolve and add activated carbon, filter to form solution A;
[0020] (2) Take 200 ml of water for injection and place it in another container, slowly add 900 mg of omeprazole sodium, and stir until the omeprazole sodium is completely dissolved to form solution B;
[0021] (3) Add solution B to solution A, add water for injection to 1000ml, add activated carbon again, stir and filter to form solution C;
[0022] (4) Detect the content of omeprazole sodium in solution C, and the qualified solution can be packaged after fine filtration, potting and sterilization to form a pharmaceutical composition injection of omeprazole sodium.
Embodiment 2
[0024] A kind of pharmaceutical composition of omeprazole sodium compound, described pharmaceutical composition is injection, and described injection is prepared by the following steps:
[0025] (1) Take 400ml of water for injection and put it in a container, then add 100mg of sodium glutamate, 10mg of sodium tartrate and 120mg of vitamin C, dissolve and add activated carbon, filter to form solution A;
[0026] (2) Take 100 ml of water for injection and place it in another container, slowly add 620 mg of omeprazole sodium, and stir until the omeprazole sodium is completely dissolved to form solution B;
[0027] (3) Add solution B to solution A, add water for injection to 1000ml, add activated carbon again, stir and filter to form solution C;
[0028] (4) Detect the content of omeprazole sodium in solution C, and the qualified solution can be packaged after fine filtration, potting and sterilization to form a pharmaceutical composition injection of omeprazole sodium.
Embodiment 3
[0030] A kind of pharmaceutical composition of omeprazole sodium compound, described pharmaceutical composition is injection, and described injection is prepared by the following steps:
[0031] (1) Take 400ml of water for injection and place it in a container, then add 180mg of sodium glutamate, 20mg of sodium tartrate and 100mg of vitamin C, dissolve and add activated carbon, filter to form solution A;
[0032] (2) Take 150 ml of water for injection and place it in another container, slowly add 700 mg of omeprazole sodium, and stir until the omeprazole sodium is completely dissolved to form solution B;
[0033] (3) Add solution B to solution A, add water for injection to 1000ml, add activated carbon again, stir and filter to form solution C;
[0034] (4) Detect the content of omeprazole sodium in solution C, and the qualified solution can be packaged after fine filtration, potting and sterilization to form a pharmaceutical composition injection of omeprazole sodium.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com