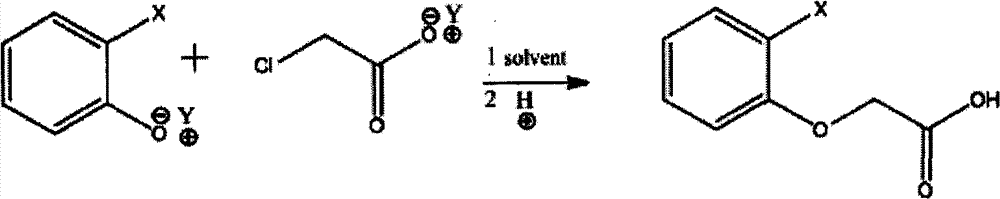
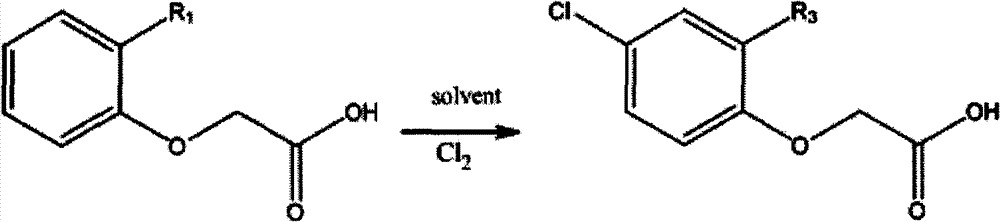
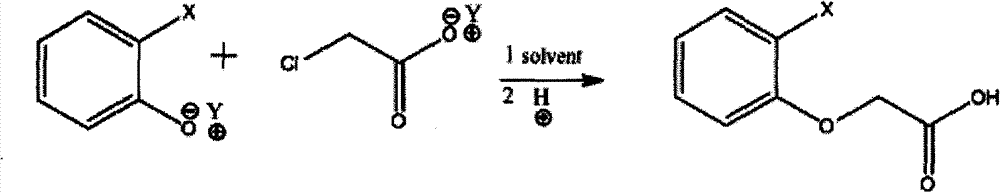
Method for synthesizing phenoxyacetic acid derivative
A technology of phenoxyacetic acid derivatives and phenoxyacetic acid, applied in the field of synthesizing phenoxyacetic acid derivatives, can solve problems such as product decomposition and uneconomical efficiency, and achieve the effects of increasing reaction production capacity, improving production efficiency and reducing manual labor.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Synthesis of Phenoxyacetic Acid
[0038]In a 1000 milliliter four-neck flask equipped with stirring, thermometer, reflux condenser and dropping funnel, add 400 grams of phenol sodium brine solution (containing 95 grams of phenol, 1 mole, 40 grams of sodium hydroxide, 1 mole), stir Heat to 90°C, start dropwise adding 460 grams of sodium chloroacetate aqueous solution (containing 125 grams of chloroacetic acid, 1.32 moles, 53 grams of sodium hydroxide, 1.33 moles), heat to reflux, react for 2 hours, cool down to 30°C, start to drop hydrochloric acid , adjust the pH value to 0, stir and cool down to 10°C, suction filter, wash, and dry to obtain 147 grams of the product, the content is greater than 98%, and the yield is 95%.
Embodiment 2
[0040] Synthesis of 2-Methylphenoxyacetic Acid
[0041] Add 450 grams of 2-methylphenol potassium brine solution (containing 108 grams of methylphenol, 1 mole, 56 grams of potassium hydroxide, 1 mole) was heated to reflux, and 450 grams of potassium chloroacetate solution (containing 115 grams of chloroacetic acid, 1.2 moles, 70 grams of potassium hydroxide, 1.25 moles) was added dropwise, heated to reflux, reacted for 2 hours, cooled to 30 ° C, and added dropwise hydrochloric acid to adjust When the pH value reaches 1, stir and crystallize to 10°C, filter with suction, add 100 grams of water to wash, and dry to obtain 162 grams of the product, with a content of 98% and a yield of 96%.
[0042] The mother liquor and washing water were distilled on a rotary evaporator in a 1000 ml bottle, and 600 grams of 2-methylphenol-containing water (containing 0.3% of methylphenol) were steamed out, and 175 grams of potassium chloride was obtained in the bottle for drying. Add and reclaim...
Embodiment 3
[0044] Synthesis of Phenoxyacetic Acid
[0045] In a 2000 ml reaction bottle, add 800 grams of phenol sodium salt solution (containing 190 grams of phenol, 2 moles, 80 grams of sodium hydroxide, 2 moles), stir and heat to 90 ° C, and start to dropwise add 900 grams of sodium chloroacetate solution (containing 243 grams of chloroacetic acid, 2.43 moles, 100 grams of sodium hydroxide, 2.5 moles), heated to reflux, reacted for 2 hours, cooled to 50 ° C, began to drop hydrochloric acid, adjusted the pH value to 0, stirred, and transferred to a 5000 milliliter separatory funnel 3,000 grams of dichloroethane was added for extraction to obtain a solution of phenoxyacetic acid (293 grams of external standard phenoxyacetic acid in the liquid phase, yield 96%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com