Application of tanshinone IIA as inhibitor for redox function of APE1 and application of tanshinone IIA in preparation of drugs used for treating cancers
A technology of tanshinone and inhibitor, applied in drug combination, antitumor drug, pharmaceutical formulation, etc., to achieve the effect of inhibiting proliferation, improving chemosensitivity, and less toxic and side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
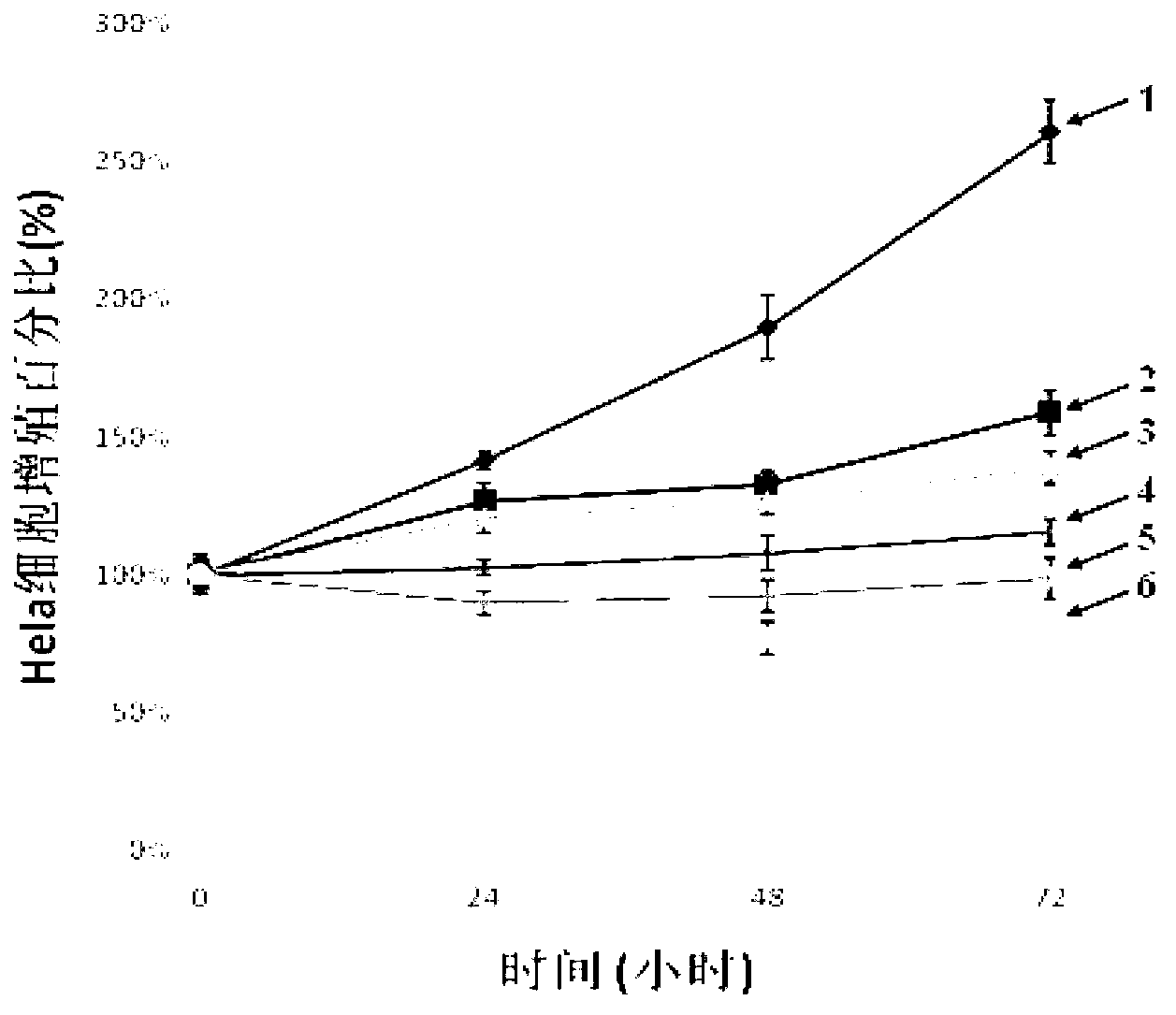
[0047] The effect of embodiment 1 Tanshinone IIA on Hela cell proliferation
[0048] Inoculate Hela cells in a 96-well plate to make the cell density about 5000-10000 / well, fill the peripheral wells with 100 μL of PBS buffer, and the temperature is 37°C, CO 2 Under the conditions of 5% volume fraction and saturated humidity, cultivate for 24 hours;
[0049]Then take the cultured Hela cells and add tanshinone IIA solution with 5 drug concentration gradients respectively (so that the final concentration is 2nmol / mL, 4nmol / mL, 8nmol / mL, 16nmol / mL, 32nmol / mL in order of molar concentration) , set 5 duplicate wells for each concentration, and take 5 wells containing Hela cells but without adding tanshinone IIA solution as a control. 2 Incubate for 24 hours at a volume fraction of 5% and saturated humidity; take another two cultured Hela cells, do the same treatment, and culture for 48 hours and 72 hours respectively;
[0050] Then, 20 μL of MTT solution with a mass-volume concent...
Embodiment 2W
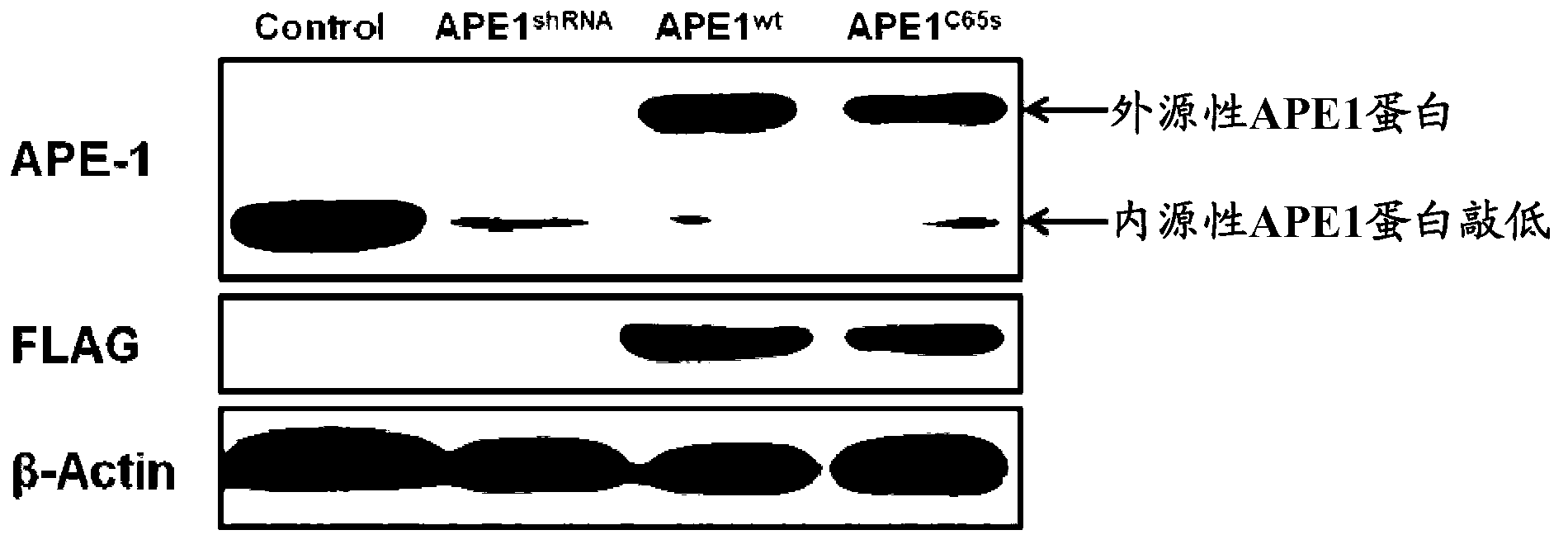
[0056] Example 2 Western Blot verification of endogenous APE1 protein knockdown and exogenous APE1 protein expression in three groups of Hela cell models
[0057] First, extract Hela cells, APE1 shRNA , APE1 wt and APE1 C65S For total cell protein, the specific operation is as follows: discard the cell culture supernatant, and then wash it twice with ice-cold PBS; add 150 μL of 1× protein loading buffer, and hang the adherent cells with a cell brush until the liquid becomes viscous; Take the viscous sample liquid into a clean 1.5mL centrifuge tube, ultrasonic 30pulse to lyse the nucleic acid until white bubbles are produced; the lysed liquid is placed on a dry thermostat for 5 minutes at a temperature of 100°C; the extracted sample is stored at -80 Store at ℃.
[0058] Then, prepare Western Blot electrophoresis gel, wherein, the lower gel consists of:
[0059]
[0060]
[0061] Prepared, after the lower layer of gel is coagulated, the concentrated gel is prepared, an...
Embodiment 3
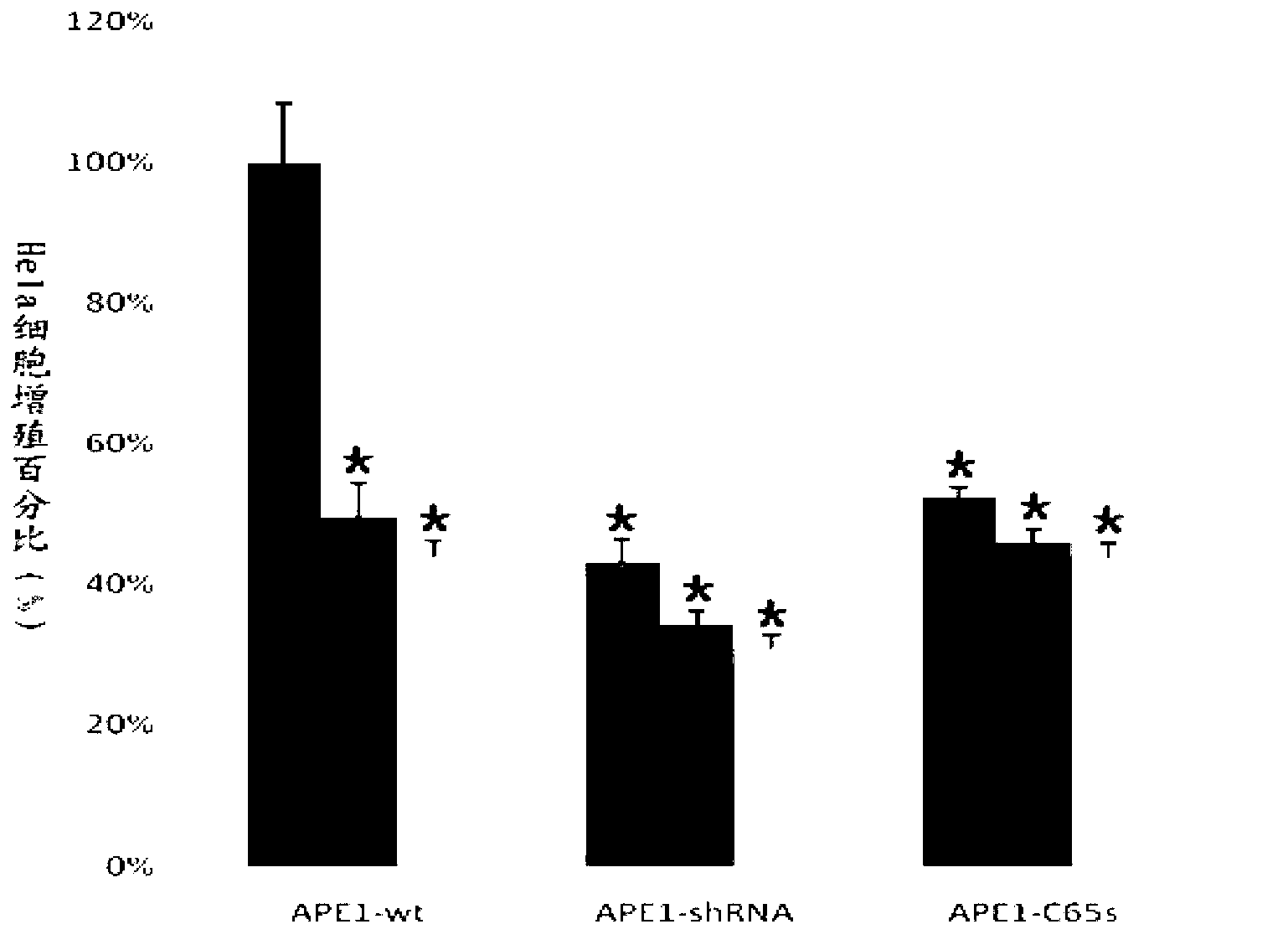
[0069] Example 3 MTT experiment to investigate the effect of APE1 stable knockdown and APE1 redox deletion on the tumor killing effect of tanshinone IIA
[0070] APE1 wt , APE1 shRNA and APE1 C65S Cells were inoculated into 96-well plates at a density of 5,000-10,000 / well; the peripheral wells were filled with 100 μL of PBS; at 37°C, CO 2 The volume fraction is 5%, cultivated for 24 hours under saturated humidity;
[0071] in APE1 wt , APE1 shRNA and APE1 C65S Two tanshinone IIA solutions with drug concentration gradients were added to the cells (to make the final concentrations respectively 8 nmol / mL and 16 nmol / mL in terms of molar concentration), and 5 replicate wells were set up for each concentration. At 37°C, CO 2 The volume fraction was 5%, and cultured for 24 hours under saturated humidity.
[0072] Add 20 μL of MTT solution with a mass-volume concentration of 5 mg / mL to each well, and continue culturing for 4 hours; terminate the culture, and carefully suck of...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com