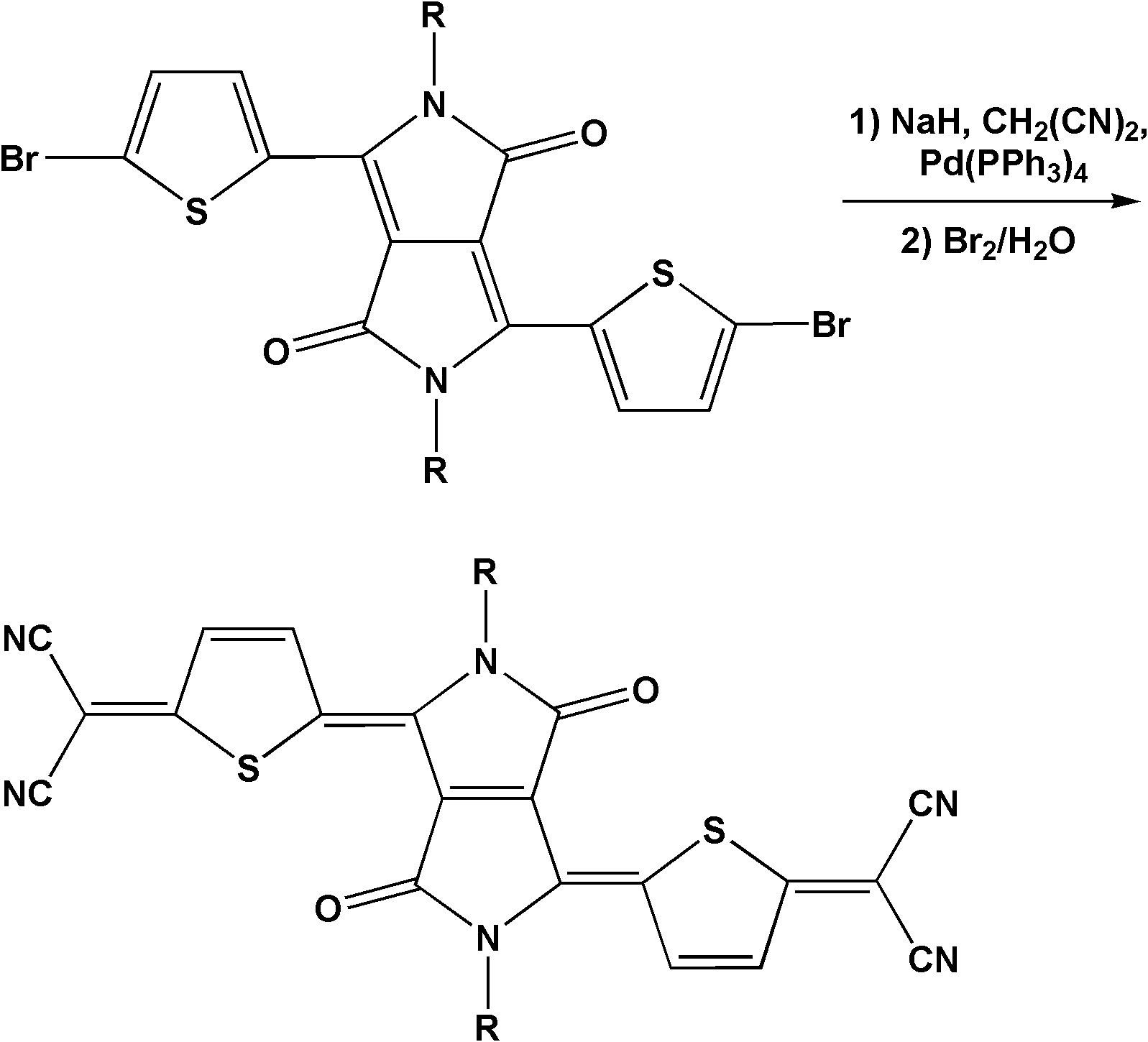
Pyrrolodione-thiophene quinone compound, and preparation method and application thereof
A technology of diketopyrrole and quinone compounds, which is applied in the field of organic semiconductor materials, and achieves the effects of simple synthesis method, low cost and improved order
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Embodiment 1: compound (a) shown in synthetic formula I and preparation n-type organic field effect transistor
[0038]
[0039] 1) compound (a) shown in synthetic formula I:
[0040] Under nitrogen protection conditions, sodium hydride (0.111g, 4.63mmol) was suspended in ethylene glycol dimethyl ether (10mL), the temperature was lowered to 0°C, and malononitrile (0.092g, 1.39mmol) was slowly added In the system, after the addition, stir at this temperature for 10 minutes, remove the ice bath, warm up to room temperature, and stir for 30 minutes. Add successively to the system the α-bromosubstituted diketopyrrole-thiophene oligomer precursor (0.393g, 0.58mmol) and tetrakis(triphenylphosphine) palladium (0 ) (0.067g, 0.058mmol), heated to reflux (temperature is 100°C). With the increase of reflux time, a dark purple precipitate was precipitated in the system after 1 hour, indicating the formation of a dianion intermediate. After 4.5 hours, cool down to room tempera...
Embodiment 2
[0050] Embodiment 2: compound (b) shown in synthetic formula I and preparation n-type organic field effect transistor
[0051]
[0052] 1) Compound (b) shown in synthetic formula I:
[0053] Under nitrogen protection conditions, sodium hydride (0.111g, 4.63mmol) was suspended in ethylene glycol dimethyl ether (10mL), the temperature was lowered to 0°C, and malononitrile (0.092g, 1.39mmol) was slowly added In the system, after the addition, stir at this temperature for 10 minutes, remove the ice bath, warm up to room temperature, and stir for 30 minutes. Add successively to the system the α-bromo-substituted pyrrole diketop-thiophene oligomer precursor (0.522g, 0.58mmol) and tetrakis(triphenylphosphine) palladium (0 ) (0.067g, 0.058mmol), heated to reflux (temperature is 100°C). With the increase of reflux time, a dark purple precipitate was precipitated in the system after 1 hour, indicating the formation of a dianion intermediate. After 4.5 hours, cool down to room temp...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com