Novel amoxicillin clavulanic acid composition
A technology of amoxicillin-clavulanic acid and clavulanic acid, which is applied in the field of novel amoxicillin-clavulanic acid compositions, can solve the problems of easy deterioration of drugs, low encapsulation rate, increased economic burden on patients, etc., and achieves reduction in production and the effect of treatment cost, dose reduction, and adverse reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
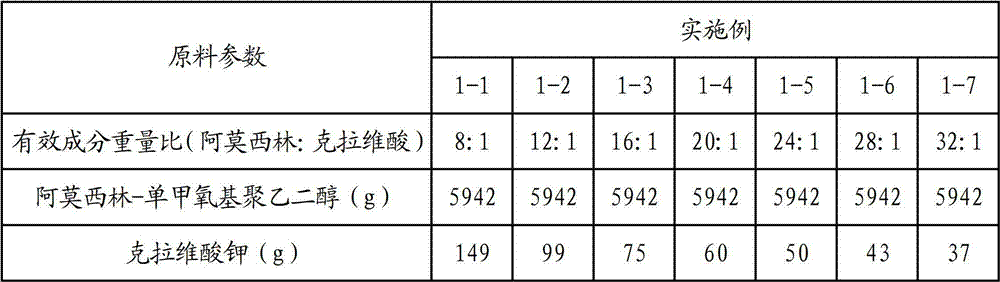
[0032] Embodiment 1 Amoxicillin-monomethoxy polyethylene glycol clavulanic acid composition
[0033] One, the preparation of amoxicillin-monomethoxypolyethylene glycol
[0034] The reaction scheme of pegylation of amoxicillin:
[0035]
[0036] (1) Preparation of aminated monomethoxy polyethylene glycol (mPEG-NH 2 )
[0037] Add 1.5L of 1M dichloromethane solution of azide acid into 10L of 896g (0.5mol) monomethoxy polyethylene glycol (mPEG) tetrahydrofuran solution, and then add 0.3L (1.5mol) diisopropyl azodicarboxylate 2 L of tetrahydrofuran solution of ester (DIAD), and 5 L of tetrahydrofuran solution of 787 g (3.0 mol) triphenylphosphine was added to the obtained mixture, stirred at room temperature for 30 min, heated at 65 °C for 2 h, and finally added with 1 L of 1M hydrochloric acid, and heated at 65 °C Heating for another 2 hours, cooling to room temperature, filtering, and vacuum drying, the yield was 99%.
[0038] (2) Preparation of amoxicillin-monomethoxypol...
Embodiment 2
[0046] Embodiment 2 amoxicillin sodium clavulanic acid-glucuronate composition
[0047] The composition involved in this embodiment is composed of unmodified amoxicillin and modified clavulanic acid, wherein amoxicillin as the active ingredient is amoxicillin sodium, and its preparation method is as follows:
[0048] 1. Preparation of clavulanic acid-glucuronate
[0049] Choose to modify the hydroxyl group of clavulanic acid instead of the carboxyl group, mainly because the isomerized carboxyl group has a certain steric hindrance, and the carboxyl group is not easy to decompose the active ingredient after entering the body after forming an ester, so that it cannot achieve good drug effect. , and the product modified by the hydroxyl group can release the active ingredient rapidly. In addition, in order to improve the esterification activity of the carboxyl group of glucuronic acid, the carboxylic acid is first converted into an acid chloride, and then esterified with the hydro...
Embodiment 3
[0065] The preparation of embodiment 3 amoxicillin-monomethoxypolyethylene glycol and clavulanic acid-glucuronate
[0066] Among the pharmaceutical ingredients involved in this example, the active ingredients are amoxicillin and clavulanic acid in modified form.
[0067] One, the preparation of amoxicillin-monomethoxypolyethylene glycol
[0068] The method in Example 1 was used to complete the preparation of amoxicillin-monomethoxypolyethylene glycol.
[0069] 2. Preparation of clavulanic acid-glucuronate
[0070] The preparation of clavulanic acid-glucuronate was completed by the method in Example 2.
[0071] The reactions involved in the above are all carried out under anhydrous conditions, and the reagents used have also been anhydrous treated in advance.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com