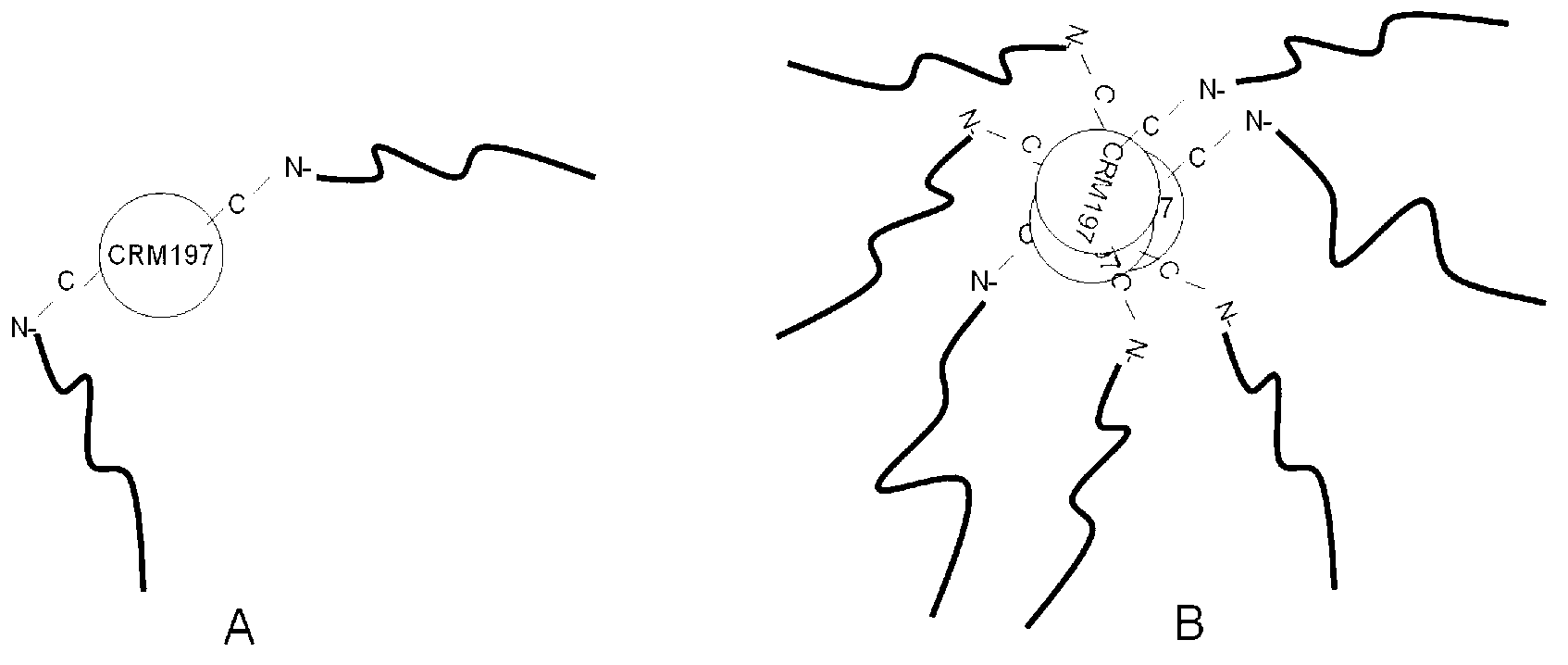
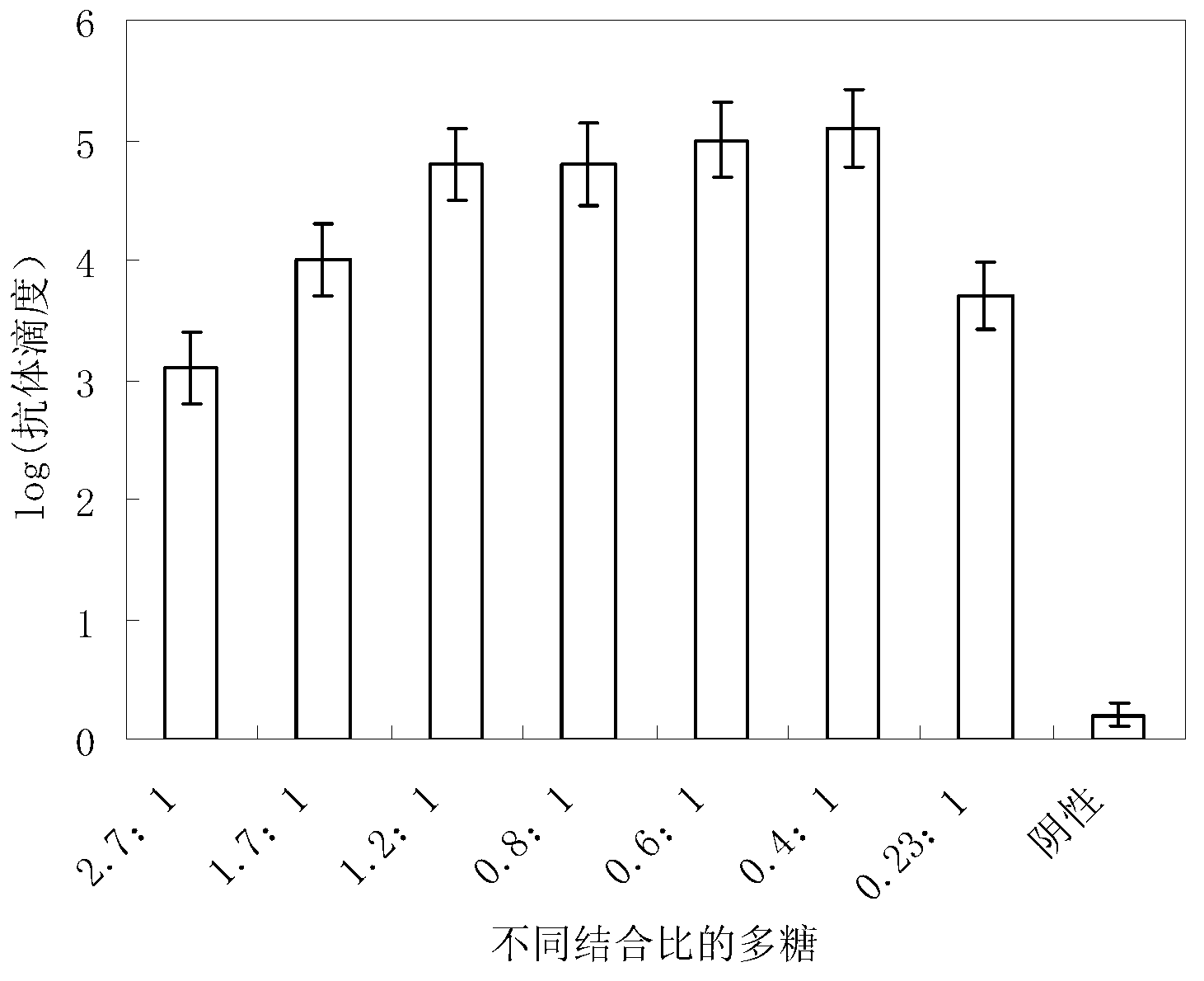Group-A neisseria meningitides capsular polysaccharide conjugated vaccine and preparation method
A Neisseria and capsular polysaccharide technology, which is applied in the direction of antibacterial drugs, pharmaceutical formulas, and medical preparations of non-active ingredients, etc., can solve the problems of inapplicable vaccines, reduce vaccine immunogenicity, etc., and achieve low production costs , Improve quality stability, simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Production of serogroup A meningococcal polysaccharide:
[0032]The meningitis capsular polysaccharide production strain (Neisseria meningitides) of group A was purchased from the China Medical Culture Collection and Management Center (strain number: CMCC (B) 29201). Neisseria meningococcus group A was fermented separately in a 50L fermenter. The obtained 30L fermentation broth was centrifuged to obtain supernatant and placed in a stainless steel tank, and concentrated to 3L by ultrafiltration using 100KD membrane bags, and adding 300ml of hexadecyltrimethyl bromide with a mass concentration of 10% to the concentrated solution Aqueous ammonium solution, stirred evenly, and then kept at 4°C for 2 hours. The quiescent solution was centrifuged to obtain a precipitate, to which was added sterile 1 M aqueous sodium chloride solution to 1 L, and dissolution of the precipitate was effected at 40°C. Add anhydrous ethanol to the lysate to a final volume concentration of 25%, s...
Embodiment 2
[0035] Preparation of carrier protein
[0036] The production strain Corynebacterium diphtheriae was purchased by the American Culture Collection Center (ATCC), and the strain number is 39255. The lyophilized diphtheria CRM197 protein-producing seeds were inoculated into test tubes containing culture medium for 16 hours. Transfer an aliquot of the culture to a 0.5 L shake flask containing growth medium and incubate the flask on a rotary shaker at 34.5-36.5°C for 8 hours. liter flask and incubate the flask on a rotary shaker at 34.5-36.5°C for 18 hours. A fermentor containing 30 L of growth medium was inoculated with this 4 liter shake flask culture. The fermenters were incubated at 30-36.5°C, pH 7.4 for 28 hours. The fermenter contents were filtered through a centrifuge and depth filter into a collector.
[0037] The obtained 30L fermentation broth was concentrated to 2L with a 30KD membrane package, and pH 7.5, 500mM phosphate buffer (sodium salt) was added to the concent...
Embodiment 3
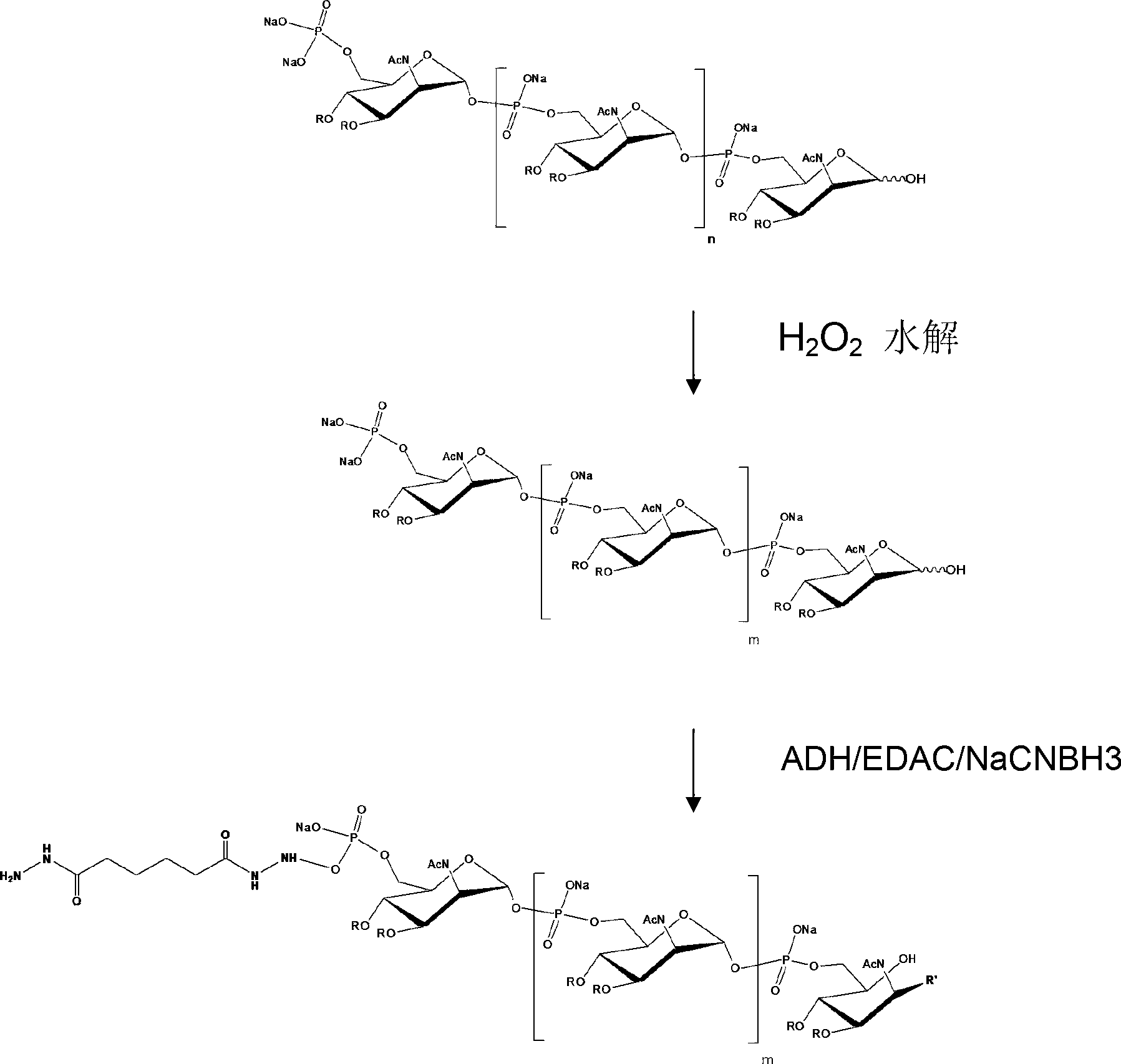
[0041] A preparation method for a group A Neisseria meningitidis capsular polysaccharide conjugate vaccine, comprising the following steps: (1) loading 2.5 g of purified group A meningococcal capsular polysaccharide powder into a reactor, adding pure water to Meningococcal capsular polysaccharide was dissolved at a temperature of 4°C when the concentration of meningoencephalic capsular polysaccharide was 4 g / L. Then, the reaction tank was heated and heated, and the meningeal capsular polysaccharide was diluted with pure water to make the concentration of 1.25 g / L for the subsequent reaction. The meningococcal capsular polysaccharide solution was heated to the set temperature according to Table 1, and 30% hydrogen peroxide was added to make the hydrogen peroxide mass concentration shown in Table 1; the temperature and reaction time were shown in Table 1, and the heater was turned off and passed through The solution was rapidly cooled to room temperature by ice-water bath circul...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com