Application of N-benzyl fatty acyl amide compound to preparation of neuroprotective drugs
A technology of benzyl fatty acid amide and neuroprotection, which is applied in drug combinations, nervous system diseases, and pharmaceutical formulations, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] The synthesis of N-benzyl fatty acid amide compounds, the synthetic route is as follows
[0027]
[0028] Fatty acid chloride is obtained by heating fatty acid and thionyl chloride under reflux. Dissolve benzylamine or benzylamine derivatives in pyridine, slowly add the obtained fatty acid chloride dropwise, and stir for 1 h. After extraction, recrystallization, etc., N-benzyl fatty acid amide compounds can be obtained.
[0029] The following N-benzyl fatty acid amide compounds were prepared in total, and the structures of the obtained compounds were respectively:
[0030] 1. N-Benzyl Palmitamide:
[0031]
[0032] 2. N-(3-methoxybenzyl)palmitamide:
[0033]
[0034] 3. N-Benzyl Octadecanamide:
[0035]
[0036] 4. N-(3-methoxybenzyl)octadecylamide
[0037]
[0038] 5. N-Benzyl Oleamide
[0039]
[0040] 6. N-(3-methoxybenzyl)oleamide
[0041]
[0042] Wherein the NMR and mass spectrometry data of compound 1 are:
[0043] 1 H-NMR (CDCl 3 )...
Embodiment 2
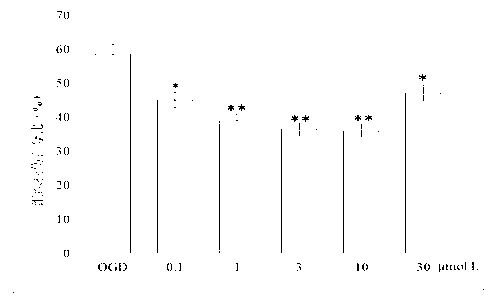
[0046] Protective effect of N-benzyl fatty acid amides on hypoxia-glucose-deficiency (OGD) injury in isolated rat brain slices
[0047] 1. Preparation of brain slices
[0048]Before the experiment, the normal cerebrospinal fluid (nACSF) was divided into two parts, which were injected with mixed oxygen at room temperature (25°C) and in an ice bath (0°C) for 1 hour to make it saturated for later use. Decapitate the rat, take out the whole brain quickly, then immerse it in ice nACSF, take it out after 1 minute, cut off along the coronal plane and flatten the telencephalon and brain at the end, paste it on the vibrating microtome bracket with 502 glue, and soak it in ice nACSF The entire brain tissue is bathed, mixed with oxygen and continuously infused. Quickly cut out a 400 μm thick brain slice, use a rough-mouthed pipette with a polished surface to move the brain slice to a watch glass, immerse in mixed oxygen-saturated ice nACSFF, quickly peel off the cortex and hippocampus,...
Embodiment 3
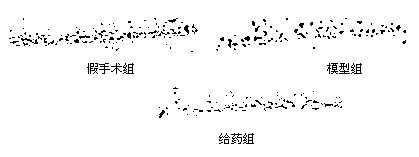
[0057] Protective effect of N-benzyl fatty acid amides on neuronal injury in transient forebrain ischemia-reperfusion gerbils
[0058] 1. animal grouping
[0059] Adult male Mongolian gerbils were fed in an environment of 22-25°C for 1-2 days before the operation, fasted the night before the operation, and had free access to water. They were randomly divided into 3 groups: sham operation group, model group and drug treatment group.
[0060] 2. Model making
[0061] The model of transient forebrain ischemia-reperfusion injury in gerbils was established by bilateral common carotid artery occlusion. Gerbils were anesthetized by intraperitoneal injection of 10% chloral hydrate solution at a dose of 350 mg / kg, and fixed in prone position. In the model group, the bilateral carotid arteries were freed, and the arterioles were clamped for 5 minutes + the arterioles were released to restore cerebral blood flow. In the sham operation group, only the bilateral carotid arteries were ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com