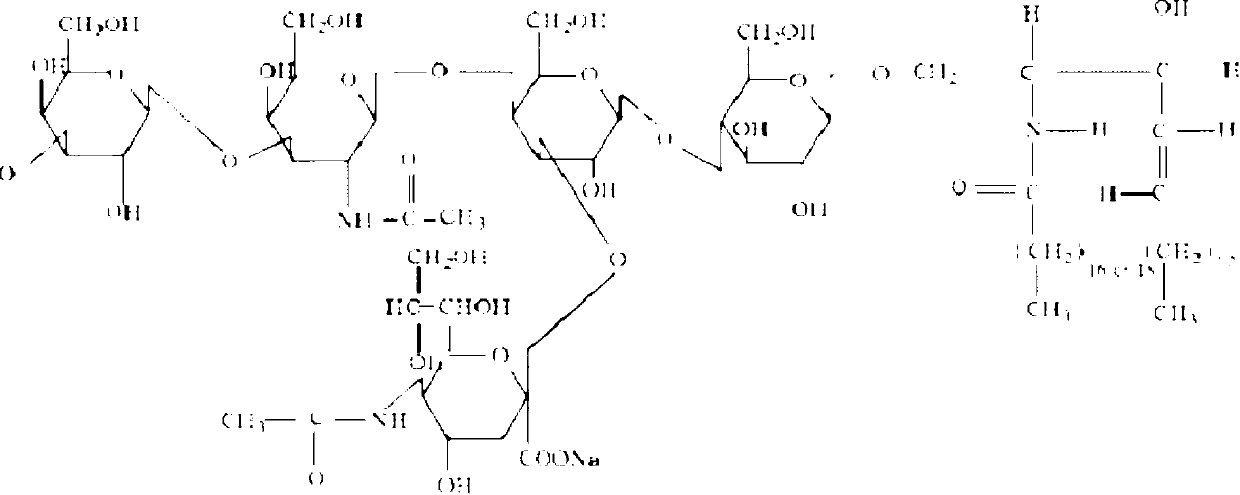
Preparation method for monosialotetrahexosyl ganglioside sodium injection
A technology of ganglioside sodium and monosialic acid, which is applied in the field of pharmaceutical preparations, can solve problems such as undefined operation process and process parameters, complicated production process, and product quality differences, and achieve good application prospects, less impurities, clarification effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Take accurately weighed disodium hydrogen phosphate 23.8g, sodium dihydrogen phosphate 4.43g, and sodium chloride 160g, add the proportioned amount of 50% (10L), stir and dissolve in water for injection at a temperature of 40°C, and then accurately weigh the Add 200 g of monosialotetrahexosylganglioside sodium raw material into the above solution at a temperature of 40° C., and stir at 900 rpm for more than 10 minutes until completely dissolved. Add 0.1% activated carbon (W / V), stir and adsorb at 900rpm for 30 minutes. Decarbonize with a 0.45μm metal filter. Add water for injection to a dosage of 95% (19L), adjust the pH value to 7.4-7.6 with a concentration of about 1.0mol / L sodium hydroxide solution or hydrochloric acid, add water for injection to make up to 20L; filter, fill, plug and cap , 121 ℃, 20 minutes rotating water bath sterilization, light inspection, packaging.
Embodiment 2
[0046] Take accurately weighed disodium hydrogen phosphate 23.8g, sodium dihydrogen phosphate 4.43g, and sodium chloride 160g, add 50% (10L) of the proportioning amount, and stir to dissolve the water for injection at a temperature of 60°C, and then dissolve the precisely weighed single Add 200 g of sialotetrahexosylganglioside sodium into the phosphate solution at a temperature of 60° C., rotate at 1000 rpm, and stir for 30 minutes until completely dissolved. Add 0.1% activated carbon (W / V), stir and adsorb at 1000rpm for 15 minutes. Decarbonize with a 0.45μm metal filter. Add water for injection to 95% of the dosage, adjust the pH value to 7.4-7.6 with a concentration of about 0.5mol / L sodium hydroxide solution or hydrochloric acid, and add water for injection to make up to 20L. Filtration, filling, plugging and capping, sterilizing in a rotating water bath at 121°C for 30 minutes, light inspection, and packaging.
Embodiment 3
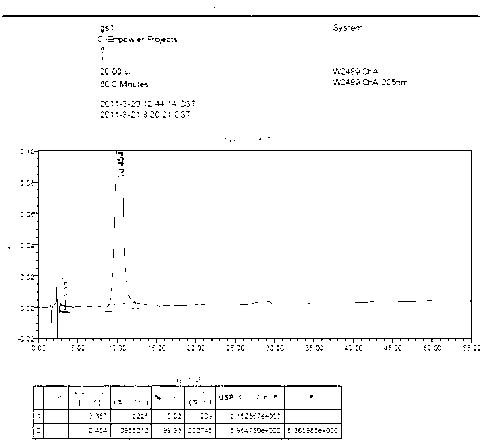
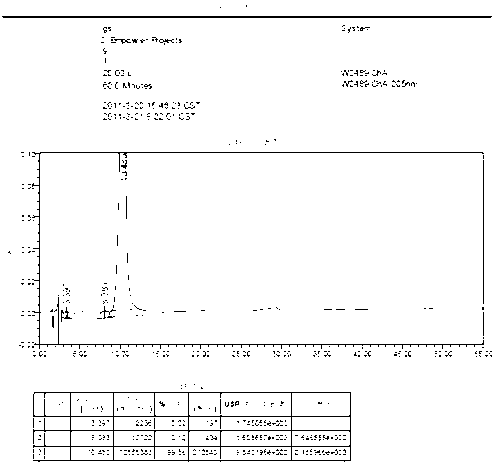
[0048] The related substances of Monosialotetrahexosylganglioside Sodium Injection are tested as follows:
[0049] HPLC method, chromatographic conditions: use amino-bonded silica gel as filler, acetonitrile-tetrahydrofuran-0.1mol / L phosphoric acid solution (66:8:34) as mobile phase, flow rate 1.0ml / min, injection volume 20μl, detection The wavelength should be 205nm.
[0050] System suitability test: Take the reference substance solution (1), the reference substance solution (2), the reference substance solution (3) and the test solution and mix them in equal amounts. Shake well as a system suitability test solution. Precisely measure 40 μl and inject it into the liquid chromatograph, and record the chromatogram. Based on the monosialotetrahexosyl ganglioside sodium peak, the number of theoretical plates should not be less than 1000, and the separation between the main peak and the adjacent impurity peaks should be greater than 1.5.
[0051]Preparation of related substance...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com