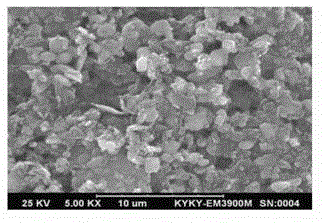
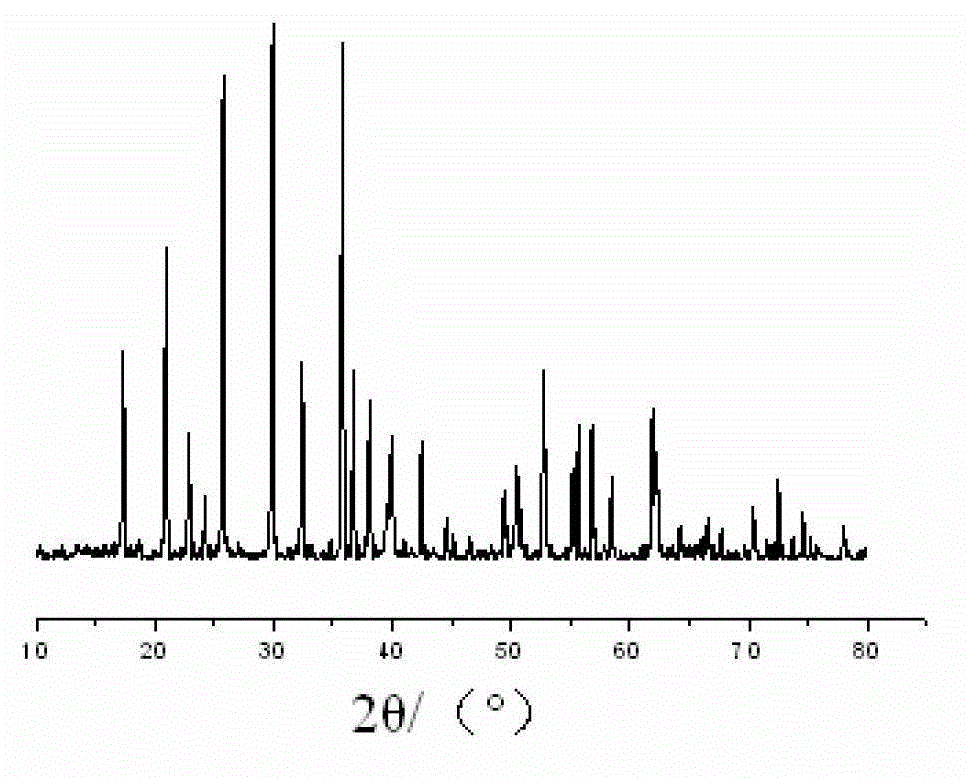
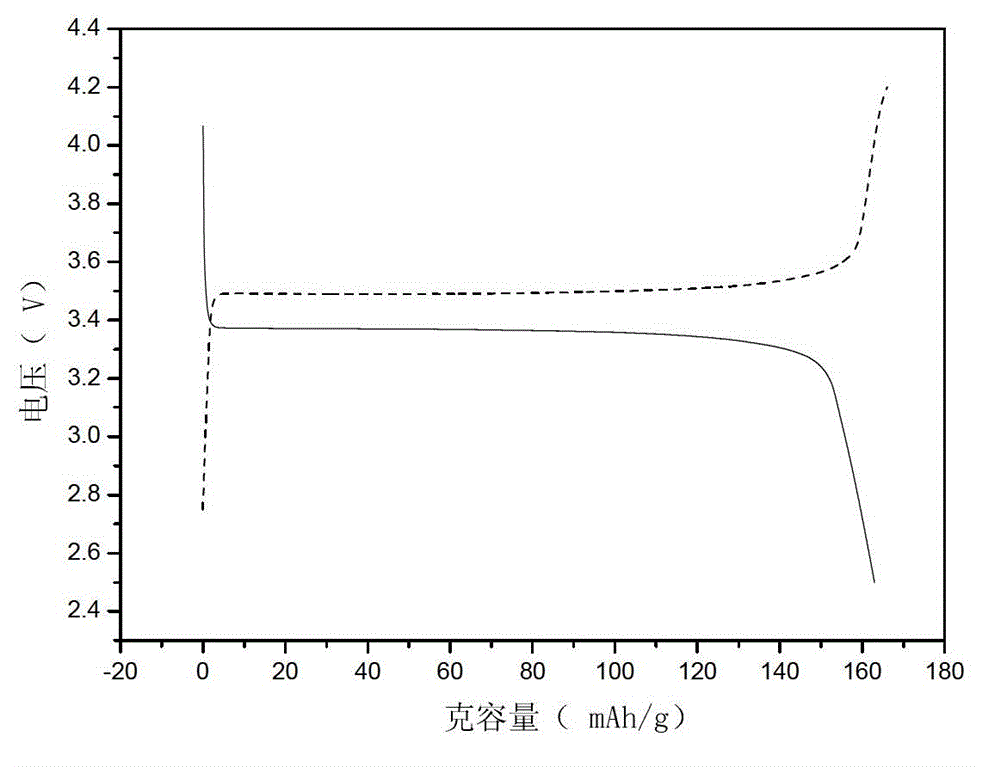
Liquid-phase co-precipitation preparation method of lithium iron phosphate cathode material
A technology of lithium iron phosphate and positive electrode materials, which is applied in the direction of battery electrodes, electrical components, circuits, etc., can solve the problems of poor charging and discharging performance of materials at high currents, difficulty in controlling product appearance, and high energy consumption in production, and achieves the goal of manufacturing The process is easy to control, the sintering time is shortened, and the cost is low
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] (1) Prepare NH with a concentration of 0.5 mol / L ferrous sulfate aqueous solution and 0.5 mol / L ammonium carbonate aqueous solution each with a pH of 7.5 3 100mL aqueous solution;
[0030] Add the above-mentioned NH into the reactor equipped with mechanical stirring, inert gas inlet and outlet and pH meter 3 200 mL of the aqueous solution was used as the mother liquor, then 0.58 g of citric acid was added as an antioxidant, and nitrogen protection was introduced. The ferrous sulfate aqueous solution and the ammonium carbonate aqueous solution were slowly added dropwise at the same time at a stirring speed of 800 revolutions / min, and the dropping speed was 2 mL / min. Fine-tune the flow rate of the two solutions to control the pH of the reaction solution to maintain between 7.5-9.5, and continue to stir and react for 0.5 hours after dripping to obtain a white ferrous carbonate precipitate; filter the ferrous carbonate precipitate and wash until it is neutral for use;
[0031] (2...
Embodiment 2
[0036] (1) Prepare 500 mL of ferrous chloride aqueous solution with a concentration of 3 mol / L and sodium carbonate aqueous solution with a concentration of 3 mol / L, NH with a pH of 8.5 3 25mL aqueous solution;
[0037] Add the above-mentioned NH into the reactor equipped with mechanical stirring, inert gas inlet and outlet and pH meter 3 The aqueous solution is used as the mother liquor, then 0.17g of oxalic acid is added, and nitrogen protection is introduced. The above-mentioned ferrous chloride aqueous solution and sodium carbonate aqueous solution are slowly added dropwise at a stirring speed of 2000 revolutions / min. The dropping rate is 0.5 mL / min. The flow rate of the solution is controlled to maintain the pH value of the reaction solution between 7.5-9.5; after dripping, continue to stir and react for 1.5 hours to obtain a white precipitate of ferrous carbonate; filter and wash the precipitate of ferrous carbonate until it is neutral for use;
[0038] (2) Quickly transfer th...
Embodiment 3
[0042] (1) Prepare 2000 mL each of ferrous nitrate aqueous solution with a concentration of 1.5 mol / L and potassium carbonate aqueous solution with a concentration of 1.5 mol / L, and NH with a pH of 9.5 3 150mL aqueous solution;
[0043] Add the above-mentioned NH into the reactor equipped with mechanical stirring, inert gas inlet and outlet and pH meter 3 The aqueous solution was used as the mother liquor, then 1.74 g of ascorbic acid was added, and nitrogen protection was introduced. The ferrous nitrate aqueous solution and the potassium carbonate aqueous solution were slowly added dropwise at a stirring speed of 1500 rpm at a rate of about 1.5 mL / min, and the two solutions were fine-tuned The flow rate control reaction solution pH value is maintained between 7.5-9.5; after dripping, continue to stir and react for 1 hour to obtain white ferrous carbonate precipitate; filter and wash the ferrous carbonate precipitate to neutral for use;
[0044] (2) Transfer the ferrous carbonate ob...
PUM
| Property | Measurement | Unit |
|---|---|---|
| electrical conductivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com