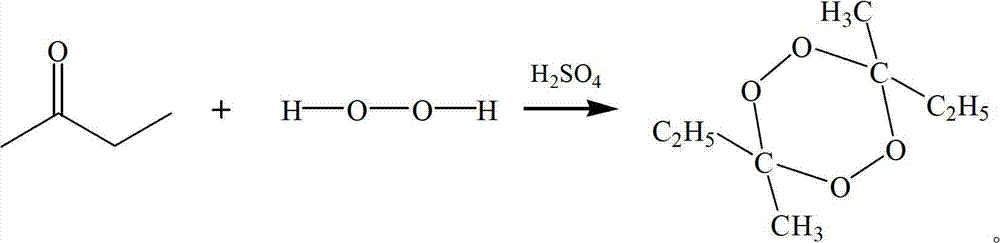
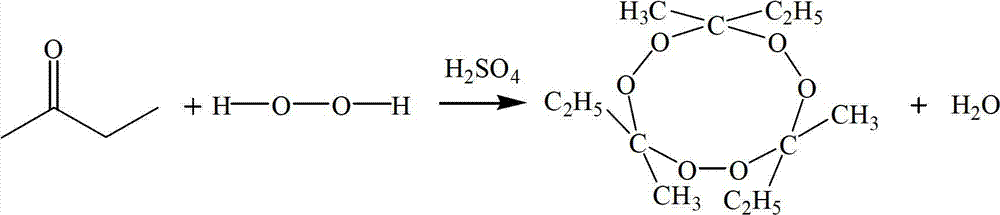Preparation method of 3,6,9-triethyl-3,6,9-trimethyl-1,4,7-triperoxynonane
A technology of triperoxynonane and trimethyl, applied in the field of organic synthesis, can solve the problems of reducing the stirring effect, reducing the effective contact of raw materials, increasing the processing capacity, etc., achieving a stable and safe production process, reducing the invalid processing capacity, and increasing the effective effect of contact
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] The preparation method of 3,6,9-triethyl-3,6,9-trimethyl-1,4,7-triperoxynonane is as follows:
[0043] (1) Add 70% hydrogen peroxide into the three-necked flask, and reduce the charging temperature to 20°C;
[0044] (2) Adjust the stirring speed to 100~200RPM, add 65% concentrated sulfuric acid dropwise to the three-necked flask, and maintain the temperature to 20°C;
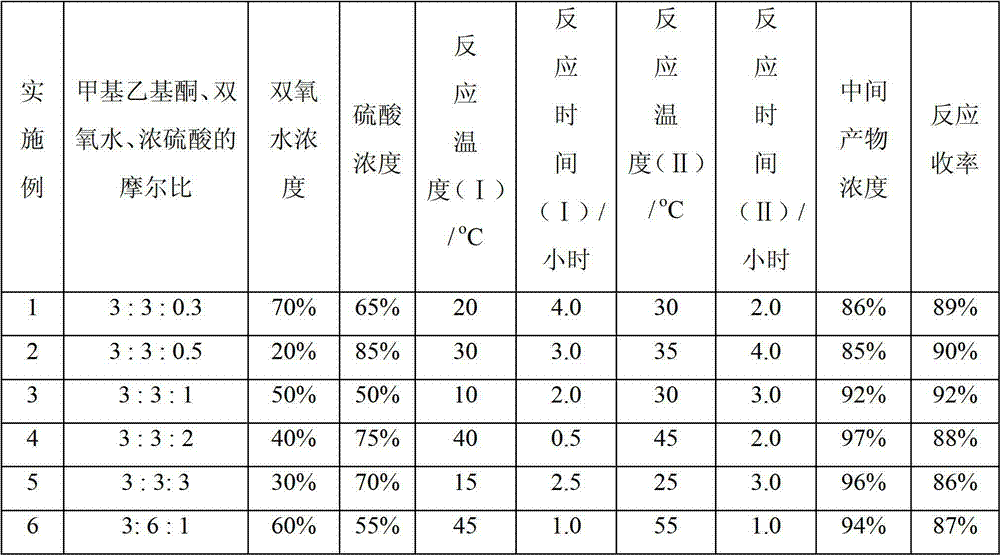
[0045] (3) Under the same stirring speed, add the calculated amount of methyl ethyl ketone dropwise to the three-necked flask, so that the molar ratio of methyl ethyl ketone, hydrogen peroxide and concentrated sulfuric acid is 3:3:0.3, and the temperature is controlled to 20 ℃, timed reaction for 4 hours, warmed up to 30 ℃, continued to react for 2 hours, the reaction was finished, cooled to 20 ℃, stood still, and layered, and the organic layer was the semi-finished product;
[0046](4) Dropwise add metered 10% sodium hydroxide solution to the above organic layer, control the reaction temperature to 20°C...
Embodiment 2
[0050] The preparation method of 3,6,9-triethyl-3,6,9-trimethyl-1,4,7-triperoxynonane is as follows:
[0051] (1) Add 20% hydrogen peroxide into the three-necked flask, and lower the feeding temperature to 30°C;
[0052] (2) Adjust the stirring speed to 100~200RPM, add 85% concentrated sulfuric acid dropwise into the three-necked flask, and keep the temperature at 30°C;
[0053] (3) At the same stirring speed, drop the calculated amount of methyl ethyl ketone into the three-necked flask so that the molar ratio of methyl ethyl ketone, hydrogen peroxide, and concentrated sulfuric acid is 3:3:0.5, and control the temperature to 30 °C, timed reaction for 3 hours, raised the temperature to 35 °C, and continued to react for 4 hours. After the reaction was completed, the temperature was lowered to 20 °C, left to stand, separated, and the organic layer was a semi-finished product;
[0054] (4) Use a metered 20% sodium hydroxide solution in the above organic layer, control the reactio...
Embodiment 3
[0058] The preparation method of 3,6,9-triethyl-3,6,9-trimethyl-1,4,7-triperoxynonane is as follows:
[0059] (1) Add 50% hydrogen peroxide into the three-necked flask, and lower the feeding temperature to 10°C;
[0060] (2) Adjust the stirring speed to 100~200RPM, add 50% concentrated sulfuric acid dropwise into the three-necked flask, and keep the temperature at 10°C;
[0061] (3) At the same stirring speed, drop the calculated amount of methyl ethyl ketone into the three-necked flask so that the molar ratio of methyl ethyl ketone, hydrogen peroxide, and concentrated sulfuric acid is 3:3:1, and control the temperature to 10 °C, timed reaction for 2 hours, raised the temperature to 30 °C, continued to react for 3 hours, the reaction was completed, cooled to 20 °C, stood still, separated, the organic layer was a semi-finished product;
[0062] (4) Add dropwise and metered 30% sodium hydroxide solution to the above organic layer, control the reaction temperature to 10°C, react...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com