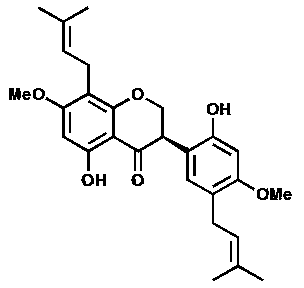
Isopentene isoflavanone compound as well as preparation method and application of compound
A technology of prenyl dihydroisoflavones and compounds, which is applied in the field of new prenyl dihydroisoflavones and their preparations, can solve the problems of poor water solubility, toxic and side effects, etc., and achieve industrial production of easy, compound The effect of high purity and good anticancer activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Compound preparation
[0036] Source of material: Wild bean was collected in Kaiyuan, Yunnan. It was identified as Uraria clarkei (Clarke) Gagnep. by researcher Tao Deding of Kunming Institute of Botany, Chinese Academy of Sciences. The specimen is preserved in the herbarium of College of Chemistry and Biology, Yunnan University for Nationalities.
[0037] Take the following steps:
[0038] a. Pulverize: dry the 10Kg wild bean stem and pulverize into coarse powder with a particle diameter of 0.1cm, for subsequent use;
[0039] b. Reflux extraction: reflux extraction of the coarse powder prepared in step a at a temperature of 70-74° C. for 4 times, each time for 2 hours, each extraction with 60 Kg of ethanol with 95% concentration, combined ethanol extract, for subsequent use;
[0040] c. Concentration: filter the ethanol extract prepared in step b through a filter paper with a pore size of 80-120 microns and concentrate under reduced pressure with a rotary evaporator a...
Embodiment 2
[0043] Example 2: Contrastive experiment of different concentrations of ethanol solutions to extract wild pea samples
[0044] Since there are not many reports on the chemical constituents of the wild pineapple, it is hoped that the optimum concentration of the ethanol solution can be found by extracting the wild pineapple samples with different concentrations of ethanol solutions. This does not imply that other solvents cannot be used for extraction.
[0045] a, pulverization: after drying the stalks of wild beans, pulverize them into a coarse powder with a particle diameter of 0.1-0.5 cm for subsequent use;
[0046]b, reflux extraction: use 6 times the ethanol of 70% concentration, 80% concentration of ethanol and 95% concentration of ethanol as the extraction solvent for the coarse powder prepared in step a at a temperature of 70-74°C, and reflux extraction for 4 Each time, each time for 2 hours, the ethanol extracts were combined for subsequent use;
[0047] c. Concent...
Embodiment 3
[0053] Structural identification of the light yellow amorphous powder obtained in Example 1
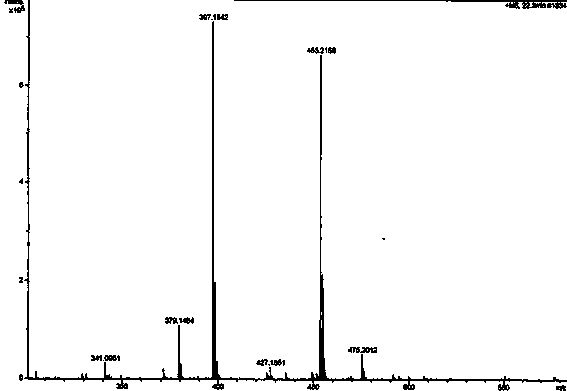
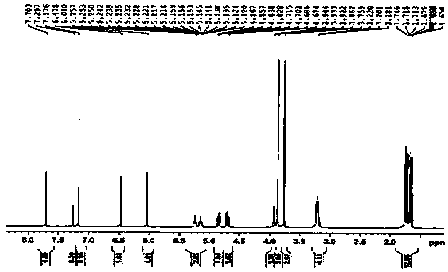
[0054] The light yellow amorphous powder (solvent is methanol), [ α ]22 D = -27.3 ( c = 0.23, MeOH); UV (MeOH) lambda max (logε): 291 (2.96) nm; CD ( c = 0.101, MeOH) λ (Δε) 258.5 (+0.435), 346 (+0.059); IR (KBr) ν max : 3360, 1640, 1601, 1507, 1453, 1274, 1108, 1010 cm -1 ; HRESI-MS (attached figure 2 ) shows its quasi-molecular ion peak m / z 453.2158 [M+H] + (calcd. 453.2232), combined with NMR spectrum to determine its molecular formula as C 27 h 32 o 6 , with an unsaturation of 12. 1 H and 13 C NMR (with image 3 , attached Figure 4 and accompanying drawings 5 , the data attribution is shown in the table- 1 ) shows that there are 2 benzene rings in the molecule (3 methine double bond carbons on the benzene ring), 2 isopentenyl groups (δ C 132.2, 131.8, 122.8, 122.1, 27.9, 25.8, 25.8, 21.0, 17.8, 17.7), 1 carbonyl (δ C 197.0), 2 methoxy groups (δ C 55.9...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com