Method for preparing boron carbon nitride nanotube with high oxygen reduction catalytic activity
A catalytic activity, boron carbon nitrogen technology, applied in chemical instruments and methods, physical/chemical process catalysts, chemical/physical processes, etc., can solve the problem of no oxygen reduction catalytic activity, etc., and achieve excellent oxygen reduction catalytic activity, operation Simple process and good tube shape
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Distill the analytically pure acetonitrile solvent at 65°C to obtain pure anhydrous acetonitrile, which is sealed and ready for use; in a nitrogen-protected glove box, 2.5 grams of analytically pure NaN 3 , 2.5 grams of analytically pure NH 4 BF 4 , 0.42 g of CTAC and 13 ml of the above-mentioned pure anhydrous acetonitrile were added successively to 16 ml of benzene, stirred for 15 minutes, and the mixture was put into a stainless steel reactor with a volume of 40 ml and sealed; Under heating for 8 hours, then the reaction kettle was naturally cooled to room temperature, and the mixture was taken out. The above mixture was washed four times with absolute ethanol, dilute hydrochloric acid and distilled water successively, filtered, and then vacuum-dried at 100° C. for 6 hours to obtain directional grown boron carbon nitrogen nanotubes with high catalytic activity for oxygen reduction.
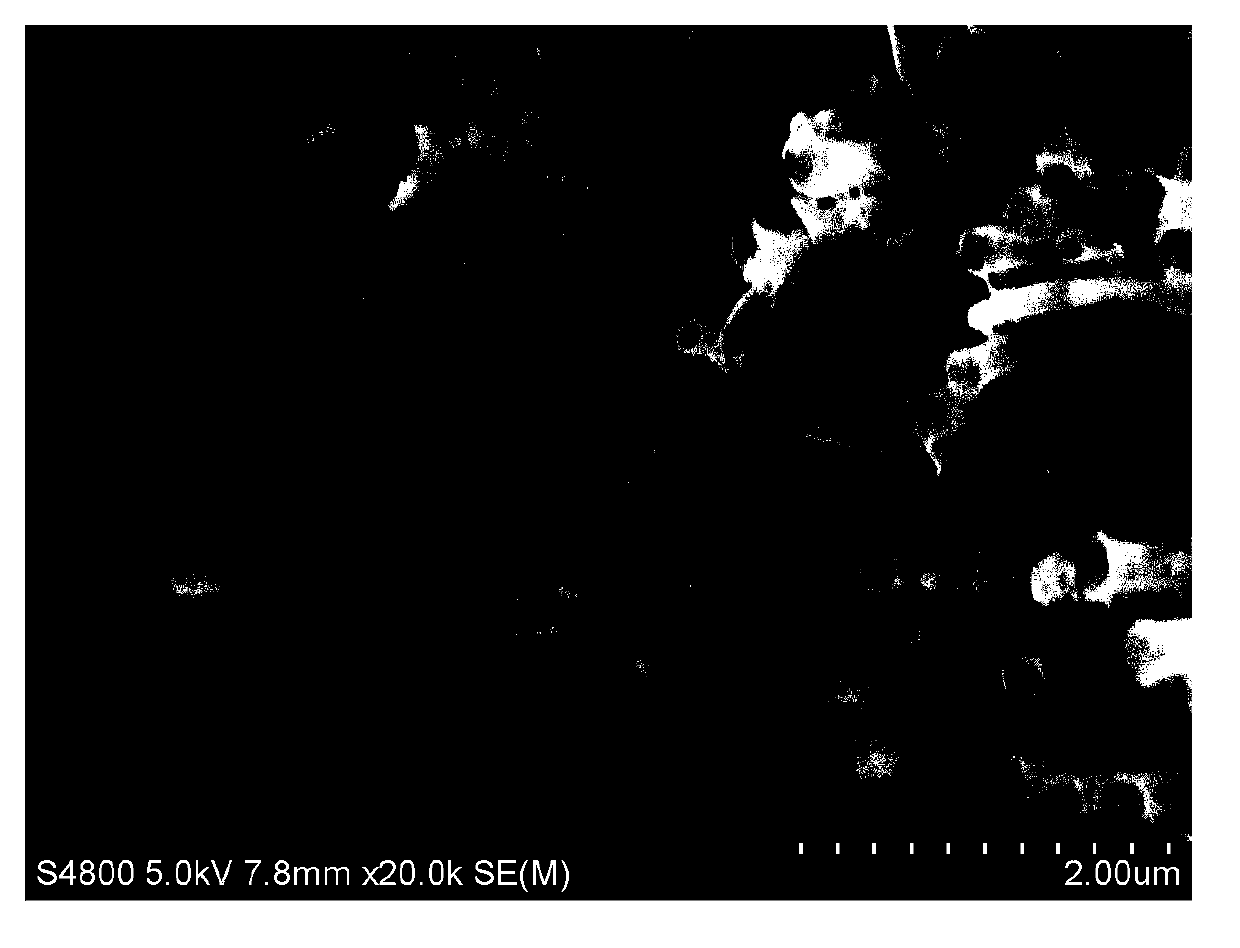
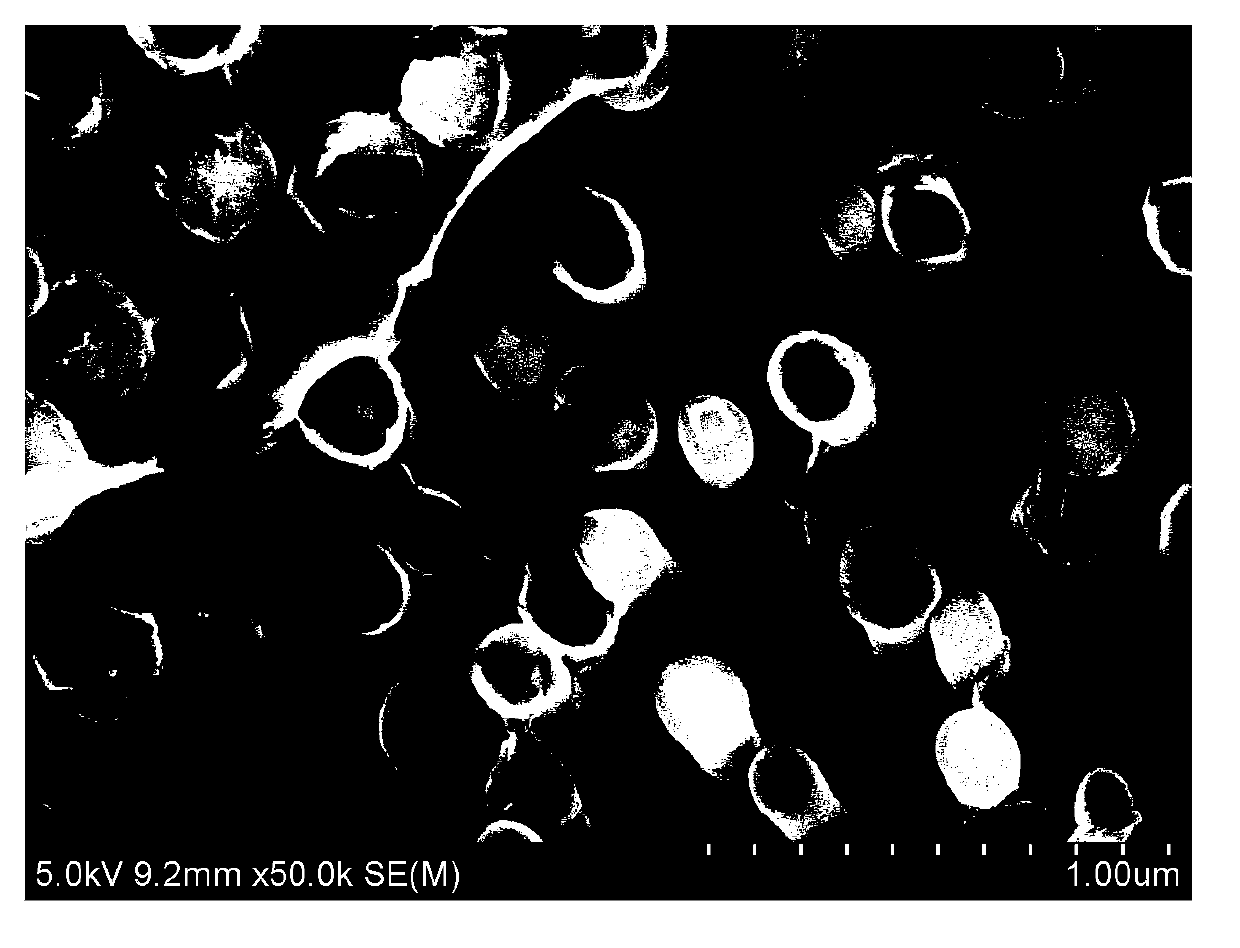
[0024] like figure 1 As shown, all nanotubes grow in the same direction, and the ...
Embodiment 2
[0028] Distill the analytically pure acetonitrile solvent at 70°C to obtain pure anhydrous acetonitrile, which is sealed and ready for use; in a nitrogen-protected glove box, 1.8 grams of analytically pure NaN 3 , 2.1 grams of analytically pure NH 4 BF 4 , 1.0 gram of CTAB and 1ml of the above-mentioned pure anhydrous acetonitrile were added successively in 8ml of benzene, stirred for 25 minutes, the mixture was put into a stainless steel reaction kettle with a volume of 15ml, and sealed; Under heating for 36 hours, then the reaction kettle was naturally cooled to room temperature, and the mixture was taken out. The above mixture was washed three times successively with absolute ethanol, dilute hydrochloric acid and distilled water, filtered, and then vacuum-dried at 60° C. for 10 hours to obtain directional grown boron carbon nitrogen nanotubes with high catalytic activity for oxygen reduction.
[0029] like Figure 4 As shown, all the nanotubes grow in the same direction,...
Embodiment 3
[0034] Distill the analytically pure acetonitrile solvent at 75°C to obtain pure anhydrous acetonitrile, which is sealed and ready for use; in a nitrogen-protected glove box, 3.0 grams of analytically pure NaN 3, 4.5 grams of analytically pure NH 4 BF 4 , 0.5 gram of CTAC and 4ml of the above-mentioned pure anhydrous acetonitrile were added successively in 24ml of benzene, stirred for 30 minutes, the mixture was put into a stainless steel reaction kettle with a volume of 40ml, and sealed; Under heating for 24 hours, then the reaction kettle was naturally cooled to room temperature, and the mixture was taken out. The above mixture was washed 5 times with absolute ethanol, dilute hydrochloric acid and distilled water successively, filtered, and then vacuum-dried at 80° C. for 8 hours to obtain directional grown boron carbon nitrogen nanotubes with high catalytic activity for oxygen reduction.
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
| lattice spacing | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com