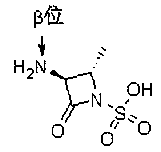
Synthetic method of aztreonam intermediate
An intermediate, aztreonam main ring technology, applied in the field of synthesis of aztreonam intermediates, can solve the problems of expensive palladium carbon, strong corrosion of instruments, and high industrialization costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0014] A, the preparation of phenylacetyl-L-threonine amide
[0015] Add 1000 mL of methanol to the flask, cool to 0 °C, add 130 mL of thionyl chloride at 0-10 °C, then cool to 0 °C, add 59.5 g (0.5 mol) of L-threonine, then slowly rise to room temperature , stirred for 6 hours, the mixture was concentrated and distilled under reduced pressure for 0.5 hours to obtain a colorless viscous oil. Dissolve the substance in 3000 mL of methanol, cool to -5 °C, saturate the solution with ammonia gas, then remove the cooling, and the sealed flask can stand for 3 days until the basic reaction of L-threonamide methyl ester is completely detected by TLC . Then the solution was concentrated to about 1000mL, 250 mL of 3M potassium hydroxide solution was added, and the reaction solution was concentrated into a viscous solution, diluted with 500 mL of water, 73.3 mL (0.55 mol) of phenylacetyl chloride, and the resulting mixture was cooled at room temperature Stir overnight, saturate the reac...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com