Positive ion nanostructure lipid carrier, manufacturing method and application thereof
A cationic lipid and carrier technology, applied in the field of biomedicine, can solve the problems of incomplete siRNA loading, and achieve the effects of low toxicity, inhibition of expression, and high transfection efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0084] The synthesis of embodiment 1 compound VI
[0085] All solvents, raw materials and reagents are analytically or chemically pure unless otherwise specified. The anhydrous treatment of the solvent is carried out according to a conventional method.
[0086] A. Using the structure of formula I with Boc 2 O is reacted to give formula II.
[0087]
[0088]
[0089] B. Formula II is reacted under the conditions of DCC and DMAP to obtain formula III with a hydrophobic tail.
[0090]
[0091] C. Formula III removes the Boc protecting group under the condition of TFA to obtain formula IV.
[0092]
[0093] D. Formula IV reacts under certain conditions to obtain formula V with a hydrophilic head.
[0094]
[0095] E. Under the condition of TFA, formula V removes the Boc protecting group to obtain formula VI.
[0096]
[0097] F. The synthetic route, reagents and conditions are as follows:
[0098]
[0099] Reagents and conditions: a.Boc 2 O,NaOH,H 2 O,...
Embodiment 2
[0100] Embodiment 2 is used for the preparation of the liposome of nucleic acid transfection
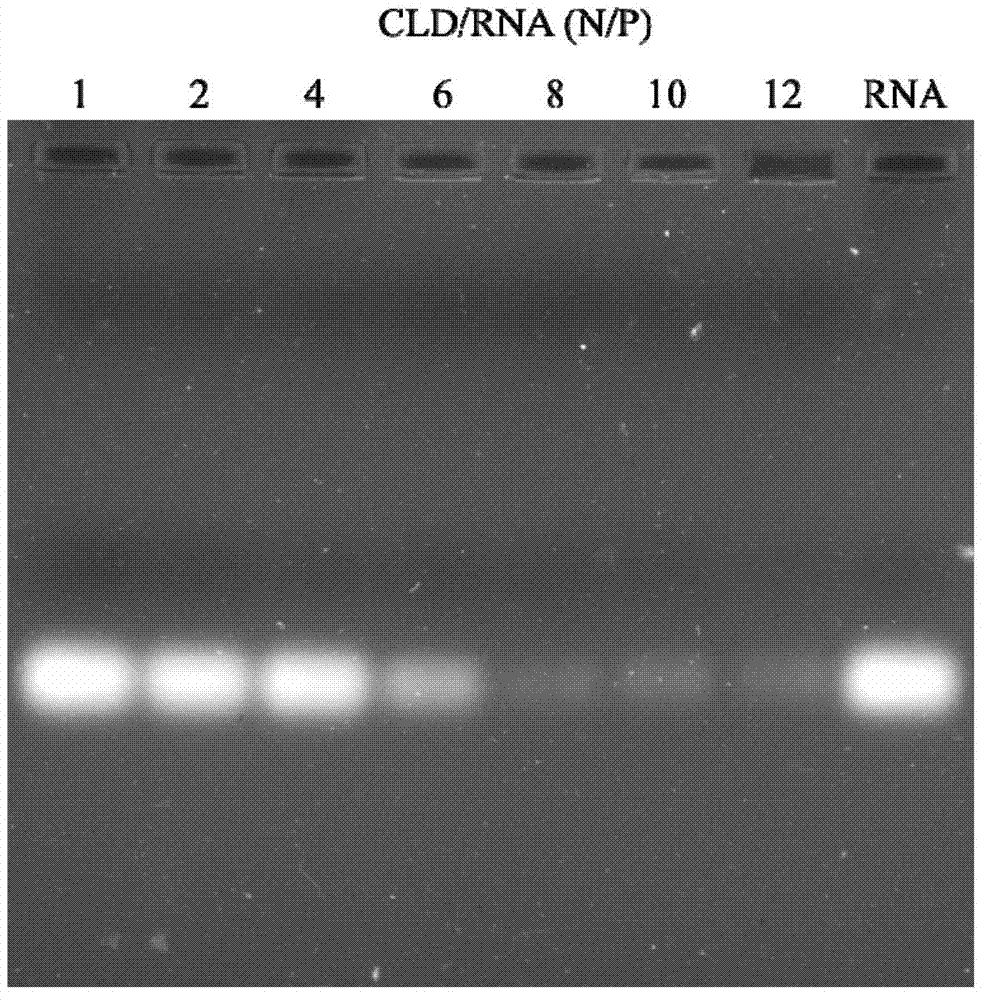
[0101] Weigh 4.2 mg of compound VI in a silanized Erlenmeyer flask, dissolve in 1 mL of organic solvent (chloroform / methanol=1:1, v / v), shake and dissolve, and dissolve at a flow rate of 5 kg / cm 2 Blow dry the solvent with nitrogen or argon gas to make the dissolved matter into a thin film. The residual solvent is slowly evaporated under reduced pressure, taken out, and dried overnight in a vacuum desiccator. Take it out the next day, add 1mL DEPC water, and seal it with a stopper. Sonicate in a water bath at 50°C for 30 minutes, and pass through a 0.25 μm sterile filter to prepare cationic liposomes (CLD).
[0102] Next, the nucleic acid solution with a concentration of 20 μM is mixed with the cationic liposome according to N / P=20, and incubated at room temperature for 30 minutes, and the preparation of the cationic liposome loaded with nucleic acid is completed.
Embodiment 3
[0103] The mensuration of embodiment 3 particle size and surface potential (Zeta potential)
[0104] The invention uses Zetaszier Nano-ZS to measure and determine the particle size and surface potential of the carrier. Results The average particle size was 119.4583nm, and the particle size distribution coefficient PDI=0.224. The surface potential is 1.72e4mv. Cationic lipid carrier particle size distribution figure of the present invention is as follows figure 1 shown.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com