Recombinant expression of actinoporins proteins
A cytolysin and sea anemone technology, which is applied in the field of recombinant expression of sea anemone cytolysin protein, can solve the problems of insufficient toxin acquisition, sea anemone marine resources and ecological environment damage.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Example 1: Construction of recombinant sea anemone cytolysin expression plasmid
[0050] Synthesize a pair of primers according to the two-terminal sequence of the mature protein encoded by the gigtIV gene and the multi-restriction site of the prokaryotic expression vector pET-22b, the upstream primer contains the Nde I restriction sequence (CATATG), and the downstream primer contains the Xho I restriction sequence (GGATCC ), the sequence is as follows:
[0051] Upstream primer: 5'AAA CATATG AGTGCTTCAGAAGTCGCTG 3' (SEQ ID NO: 3, the underlined part is the Nde I restriction sequence that is not translated)
[0052] Downstream primer: 5'TTT CTCGAG GCGTGAAATCTTAATTTGCAG 3' (SEQ ID NO: 4, the underlined part is the sequence cut by Xho I)
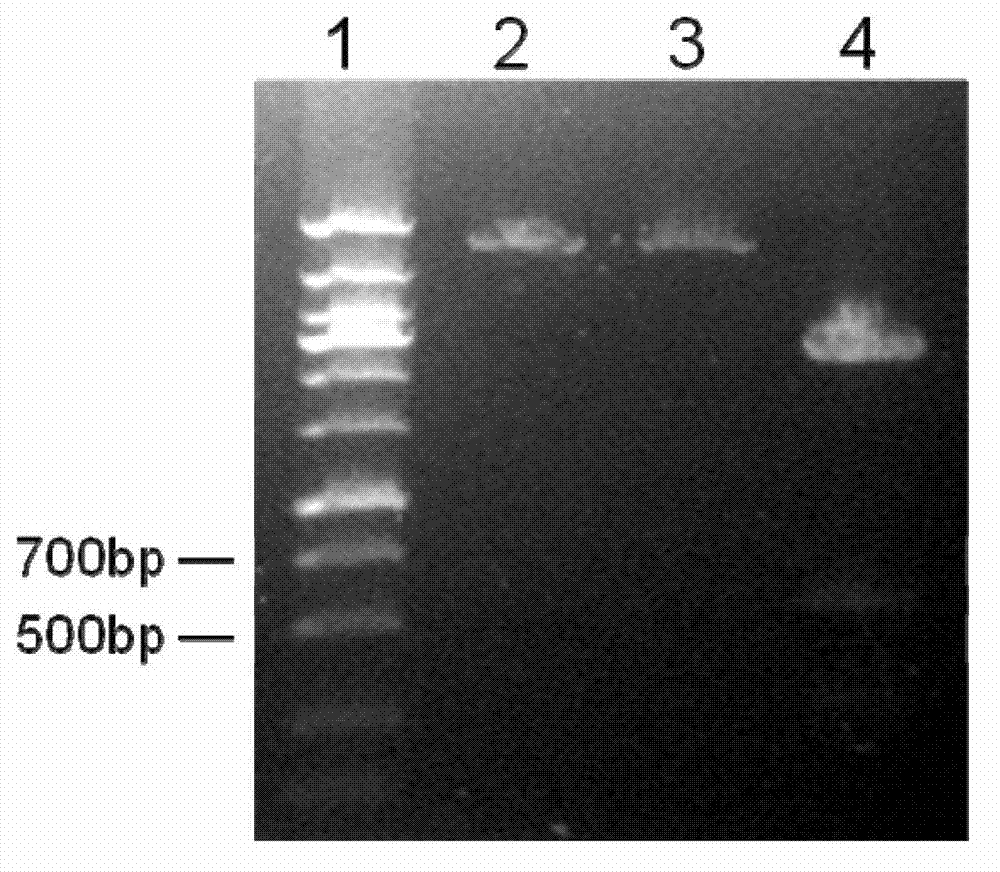
[0053] PCR amplification and gene cloning were carried out according to conventional methods. The PCR product is about 540bp (SEQ ID NO:1), encoding 179 amino acid residues (SEQ ID NO:2). The target gene was cloned into the prokaryo...
Embodiment 2
[0054] Embodiment 2: Expression of recombinant rGT-4
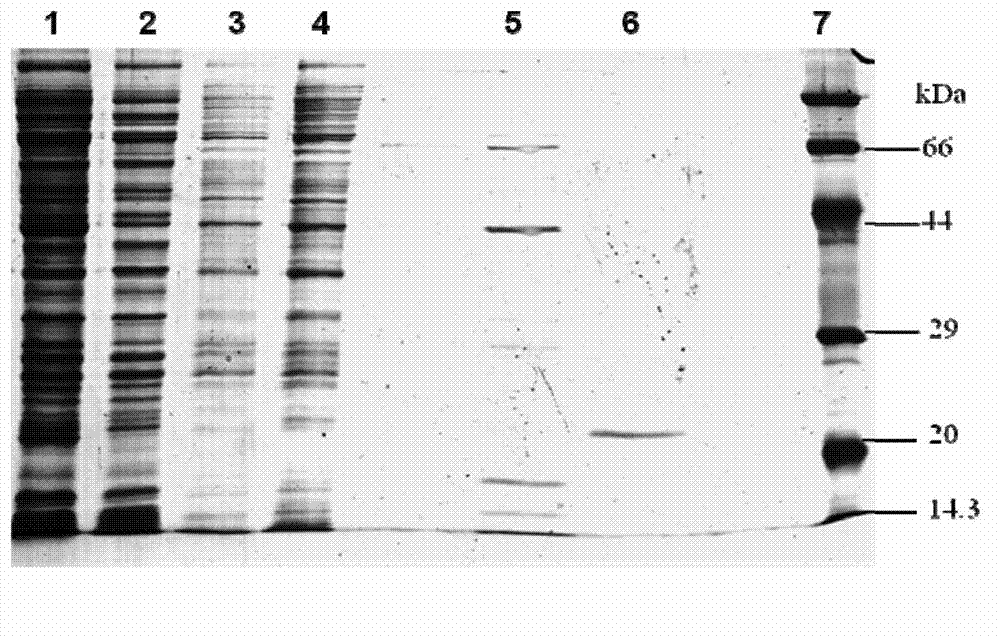
[0055] pET-22b-gigtIV was transformed into Escherichia coli BL21 (purchased from Novagen). SDS-PAGE electrophoresis analysis of the supernatant of genetically engineered bacteria sonication showed that the bacteria had obvious specific expression product bands after induction, and the molecular weight was consistent with the predicted theoretical value of 19.7kD ( figure 2 ).
[0056] After exploring the cultivation time, induction concentration, temperature and other conditions, and comparing the induced expression of the bacteria after expanding the culture for different periods of time, the final culture conditions for the genetically engineered bacteria were as follows: a single colony was inoculated in 50ml of ampicillin-resistant LB liquid medium ( 1% (w / v) tryptone, 0.5% (w / v) yeast extract, 1% (w / v) sodium chloride), culture overnight at 37°C, 180rpm, take 20ml of overnight culture and inoculate 2L Ampicillin-re...
Embodiment 3
[0057] Embodiment 3: Purification of recombinant rGT-4 protein
[0058] The harvested total bacteria were washed with PBS buffer (10mmol / L, pH 7.5), then suspended with solution A (20mmol / L PBS, 500mmol / LNaCl, 20mmol / L imidazole, pH 7.4), and after ultrasonic treatment, centrifuged ( 4°C, 12000rpm, 30min), the supernatant was Ni 2+ One-step purification by affinity chromatography.
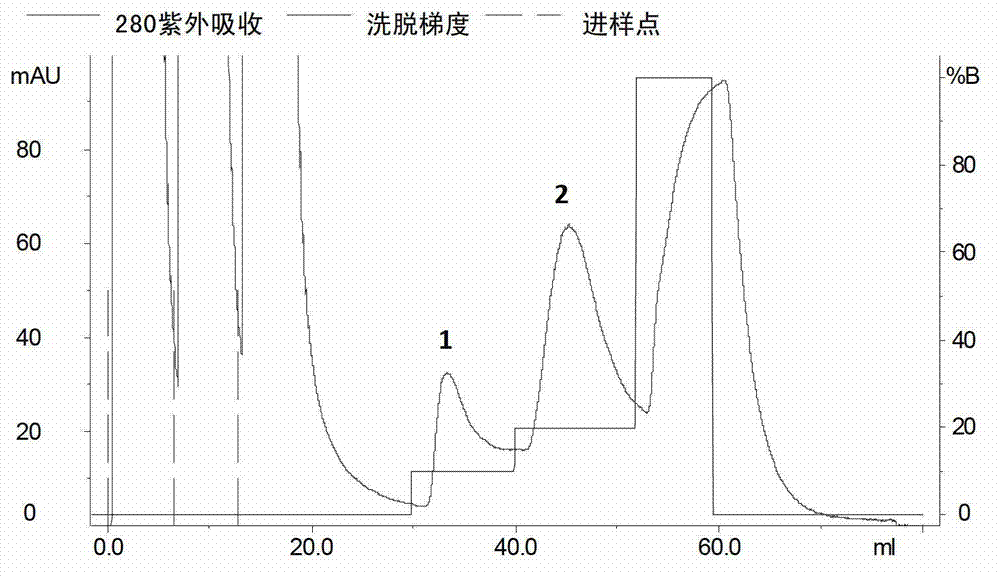
[0059] The column was pre-equilibrated with solution A. After loading the sample, use solution B (20mmol / L PBS, 500mmol / LNaCl, 500mmol / L imidazole, pH 7.4) for gradient elution, the elution time of each gradient is 10min, and the flow rate is 1ml / min. Recombinant rGT-4 was eluted in 20% B solution ( image 3 ), store rGT-4 at -75°C. Another small amount was taken for SDS-PAGE analysis, showing that its molecular weight was about 19KD and the purity reached more than 90% ( figure 2 ).
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com