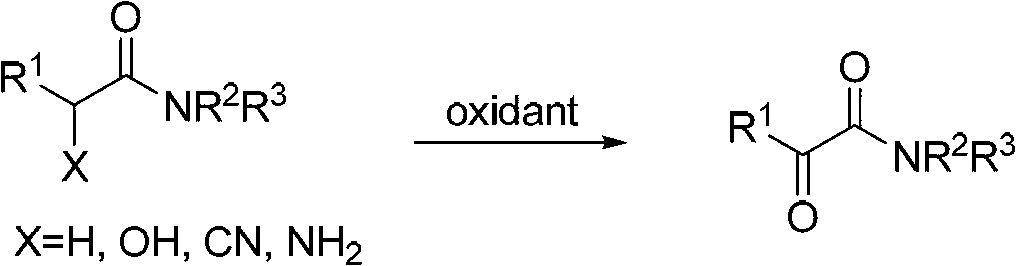
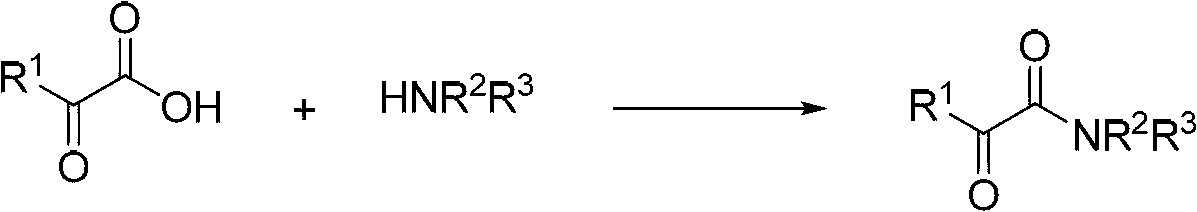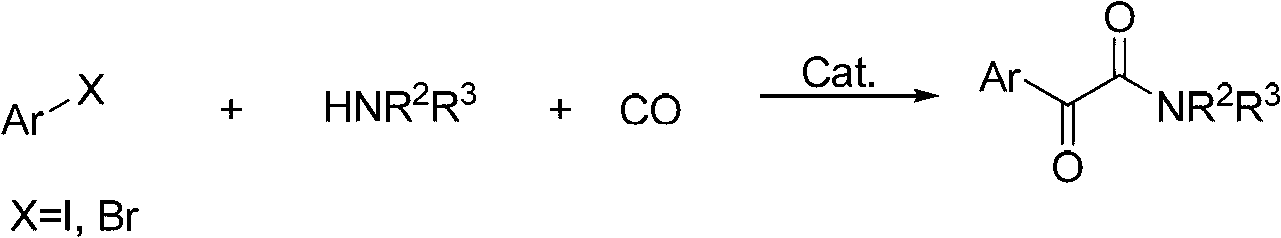Method for preparing alpha-ketoamide compounds
A technology of compound and ketoamide, which is applied in the field of preparation of α-ketoamide compounds, achieves the effects of easy post-processing, favorable large-scale industrial production, and good economy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047]
[0048] In a 25mL round bottom flask, at room temperature, add cuprous iodide (0.19g, 1mmol), acetophenone (0.59mL, 5mmol), piperidine (1.5mL, 15mmol), and then React at 50°C for 20 hours. After the reaction was completed, cool to room temperature, add 50 mL of ethyl acetate, and extract with 25 mL of water. The ethyl acetate layer was washed with dilute hydrochloric acid (0.5 M) and saturated brine, dried over anhydrous sodium sulfate, and concentrated. The concentrate was subjected to flash column chromatography (petroleum ether: ethyl acetate = 5:1) to obtain 0.96 g of the product (light yellow solid), yield: 88%. 1 H NMR (CDCl 3 ,600MHz,ppm):δ=7.97-7.95(d,J=7.4Hz,2H),7.67-7.63(t,J=7.4Hz,1H),7.54-7.51(t,J=7.7Hz,2H), 3.72(brs,2H),3.32-3.29(t,J=5.6Hz,2H),1.72-1.70(m,4H),1.56(brs,2H); 13 C NMR (CDCl 3 ,150MHz,ppm):δ=191.9,165.5,134.6,133.3,129.6,129.0,47.0,42.2,26.2,25.5,24.4; HRMS calcd for C 13 h 15 NNaO 2 (M+Na) + 240.0995, found 240.1001.
Embodiment 2
[0050]
[0051] In a 25mL round bottom flask, at room temperature, add cuprous iodide (0.048g, 0.25mmol), toluene 10mL, p-nitroacetophenone (0.83g, 5mmol), piperidine (1mL, 10mmol), and then React at 50°C for 10 hours under an atmosphere of oxygen. After the reaction was complete, concentrate under reduced pressure to remove toluene, add 50 mL of ethyl acetate, and extract with 25 mL of water. The ethyl acetate layer was washed with dilute hydrochloric acid (0.5 M) and saturated brine, dried over anhydrous sodium sulfate, and concentrated. The concentrated solution was subjected to flash column chromatography (petroleum ether: ethyl acetate = 5:1) to obtain 1.19 g of the product (light yellow solid), yield: 91%. 1 H NMR (CDCl 3 ,600MHz,ppm):δ=8.36-8.34(d,J=8.8Hz,2H),8.15-8.13(d,J=8.8Hz,2H),3.74-3.72(brd,2H),3.33-3.30(t ,J=5.5Hz,2H),1.73(brs,4H),1.59(brs,2H); 13 C NMR (CDCl 3 ,150MHz,ppm):δ=189.5,164.1,151.1,137.8,130.6,124.1,47.1,42.5,26.3,25.4,24.3; HRMS calcd for C 1...
Embodiment 3
[0053]
[0054] In a 25mL round bottom flask, at room temperature, add cuprous iodide (0.19g, 1mmol), p-chloroacetophenone (0.65mL, 5mmol), piperidine (1.5mL, 15mmol), and then React at 50°C for 20 hours under oxygen. After the reaction was completed, cool to room temperature, add 50 mL of ethyl acetate, and extract with 25 mL of water. The ethyl acetate layer was washed with dilute hydrochloric acid (0.5 M) and saturated brine, dried over anhydrous sodium sulfate, and concentrated. The concentrate was subjected to flash column chromatography (petroleum ether: ethyl acetate = 5:1) to obtain 1.03 g of the product (yellow oil), yield: 82%. 1 H NMR (CDCl 3 ,600MHz,ppm):δ=7.91-7.88(d,J=8.5Hz,2H),7.50-7.47(d,J=8.5Hz,2H),3.71-3.69(t,J=5.2Hz,2H), 3.30-3.27(t,J=5.6Hz,2H),1.73-1.67(m,4H),1.58-1.53(m,2H); 13 C NMR (CDCl 3 ,150MHz,ppm):δ=190.5,164.9,141.2,131.7,130.9,129.4,47.1,42.3,26.3,25.4,24.4; HRMS calcd for C1 3 h 14 ClNNaO 2 (M+Na) + 274.0605,found 274.0604.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com