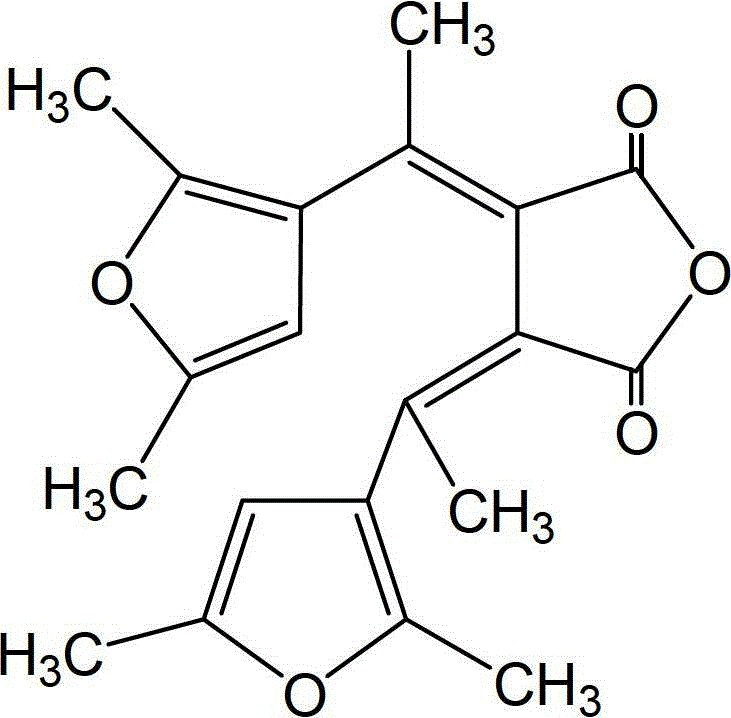
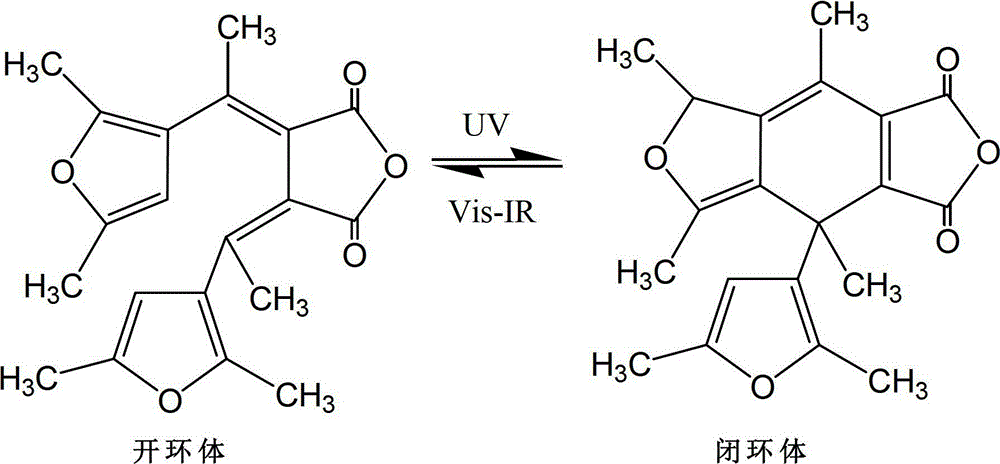
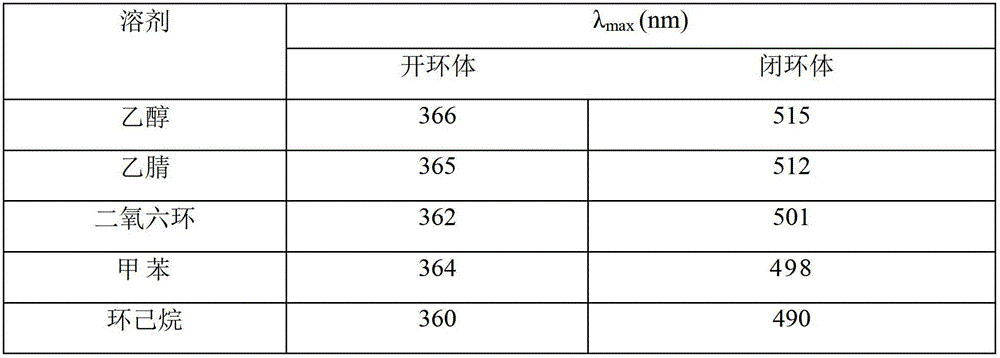
Synthetic method of dual-furan substituted fulgide photochromic compound
A technology of fugitive anhydride and photochromism, applied in chemical instruments and methods, color-changing fluorescent materials, organic chemistry, etc., can solve the problems of harsh synthesis conditions, low yield of target compounds, and difficulty in synthesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Add 15mL of anhydrous tert-butanol to a dry 250mL three-necked bottle, and then dissolve 5g of potassium metal in it to obtain a potassium tert-butoxide solution. Add 9g of 2,5-dimethyl-3-acetylfuran and 28g of butanedi Diethyl acetate and 55mL of tert-butanol were mixed to obtain a mixed solution, and the above mixed solution was added dropwise to the above-mentioned potassium tert-butoxide solution for 1 hour under stirring at 20°C. After the addition was completed, reflux was continued for 24 hours, and then cooled to room temperature, acidify with 5mol / L hydrochloric acid to pH 5-6, then distill off the solvent under reduced pressure, dissolve the residue in 200-400mL water, extract twice with ether (2×50mL), and wash the water layer with Acidify with hydrochloric acid (6mol / L) to strong acidity (PH = 1), and after static separation, the reddish-brown organic phase monoester is extracted with ether for 3 times (3×100mL), dried and filtered with anhydrous sodium sulfa...
Embodiment 2
[0034] Same as Example 1, but the consumption of step (1) 2,5-dimethyl-3-acetylfuran is changed from 9g to 11.5g, the consumption of diethyl succinate is changed from 28g to 33.6g, other Change.
Embodiment 3
[0036] Same as Example 1, but the consumption of step (1) 2,5-dimethyl-3-acetylfuran is changed from 9g to 8.2g, the consumption of diethyl succinate is changed from 28g to 23.8g, other Change.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com