Photostable cis 2, 3-cyclopropanated abscisic acid analogue and preparation method thereof
A technology of cyclopropanation and abscisic acid, which is applied to the preparation of oxygenated compounds, botany equipment and methods, chemical instruments and methods, etc., can solve the problems of poor biological activity and loss of meaning
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
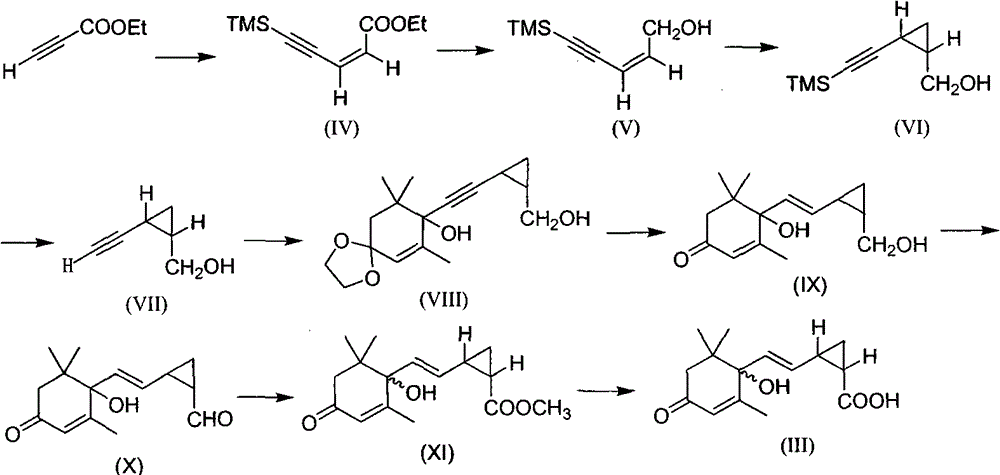
[0012] Embodiment 1: the synthesis of compound (III)
[0013] The first step: the synthesis of ethyl (Z)-5-trimethylpent-2-en-4-ynoate (IV)
[0014] In a 250 mL dry three-necked flask, 7.84 g of ethyl propiolate, 10.06 g of anhydrous lithium bromide, 5.28 g of acetic acid and 80 mL of anhydrous acetonitrile were sequentially added, stirred, and heated to reflux for 22 h. Then cool to room temperature, pass through nitrogen protection, first add solvent anhydrous triethylamine 120mL under stirring, then add trimethylsilylacetylene 10.43g, bistriphenylphosphorous palladium chloride 0.99g and cuprous iodide 0.54 g. The reaction was carried out under ice bath for 6 h. After the reaction was completed, the reaction solution was passed through a small section of silica gel decompression column, and the column was washed with ethyl acetate. The obtained filtrate was concentrated to obtain a brown liquid. Direct column chromatography (petroleum ether: ethyl acetate = 8:1) gave 12.7 ...
Embodiment 2
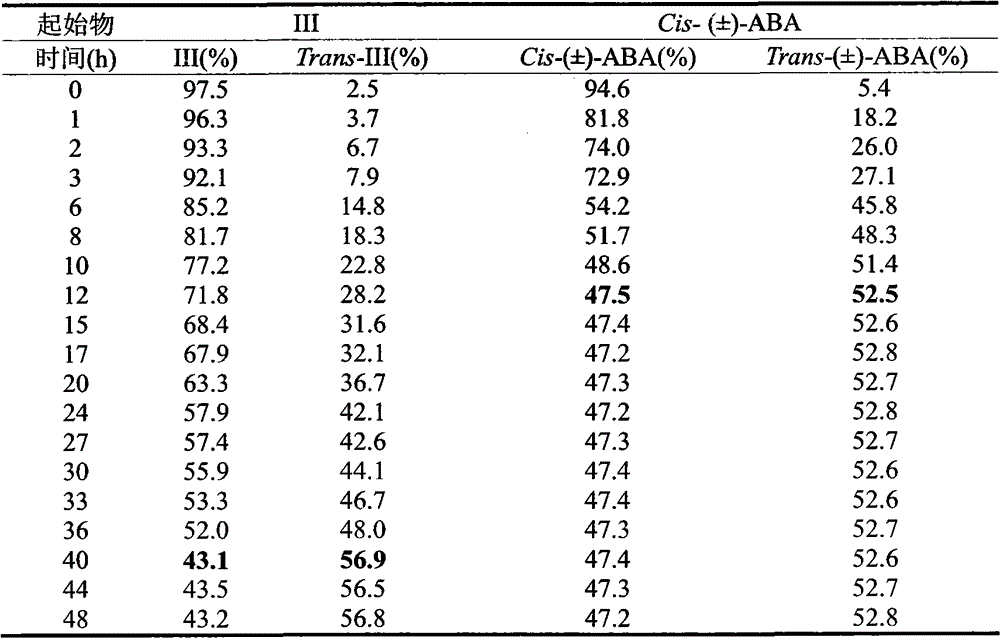
[0032] Example 2: Photostability of Cis-2,3-cyclopropanated abscisic acid analog (III)
[0033] Using a 254nm ultraviolet lamp as the light source (the light source comes from the ZF-I three-purpose ultraviolet analyzer), keep the relative position (5cm) of the sample bottle and the ultraviolet lamp constant during illumination. The structure of the isomerization product was confirmed by HPLC-MS, the photoisomerization of different conditions and compounds was evaluated by HPLC, and the analysis method of each compound was established. The area normalization method was used to determine the photoisomerization content of each compound.
[0034] Drug preparation: accurately weigh 2.6mg (1.0×10 -5 mol) compound (III), dissolved in 1 mL of methanol to obtain a 10 mmol / L stock solution of each compound, and stored at -20°C in the dark. Pipette 100 μL stock solution and dilute to 10 mL with water for light experiment. Keep the relative position (5cm) between the sample bottle and...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com