Recombinant human nerve growth factor deletion mutant, its preparation method and application
A technology for deletion of mutants and growth factors, applied in the field of mutants, can solve the problems of rhNGF quality control and other problems, and achieve the effects of maintaining the biological activity, the C-terminal of the protein is uniform, and the protein expression level is improved.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
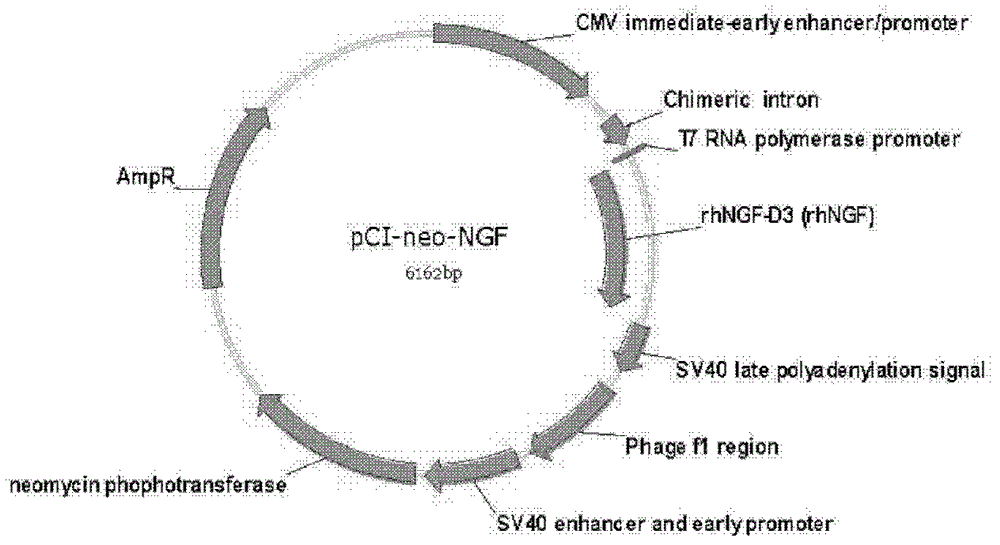
[0032] Example 1 Recombinant human nerve growth factor deletion mutant (rhNCF-D3) gene cloning and vector construction
[0033] Design primers according to the published human NGF DNA sequence (Genbank: NM_002506), isolate human peripheral blood albumin, use TriZol (purchased from Invitrogen), extract its total RNA, reverse transcribe into cDNA, reverse transcription system is template RNA 2 μg , 5× reaction solution 4μl, dNTP mixture (10mM, each) 1μl, RNase inhibitor (20U / μl), random primer 2μl, M-MLV reverse transcriptase (200U / μl) 1μl, add water to make up the total volume to 20μl. After standing at room temperature for 10 min, react at 42°C for 1 h. Using this cDNA as a template, the human NGF gene and the deletion mutant gene were amplified. The upstream primers for amplification were "5'-atgaa ttcca ccatg tccat gttgt tctac actc t ga-3'", and the downstream primers were "5'-atccc gggtt atcag gctct tctca cagcc ttcct gct-3' (for full-length NGF gene amplification)" and "5'...
Embodiment 2
[0038]The prepared pCI-neo NGF and pCI-neo NGF-D3 plasmids were electrotransformed into CHO S cells (purchased from Invitrogen Company), the electroporation apparatus was Gene pulser Xcell (BIO-RAD Company), and the electroporation conditions were 160V, 150ms. The medium is DMEM with 10% fetal bovine serum added. After electroporation, the cells in the electroporation cup are washed out with the medium, and spread in a 35mm cell culture dish. On the third day, 600 μg / ml 6418 (Sigma company) was added to the culture medium to pressurize and select resistant cells, and the surviving monoclonal cell clusters after resistance selection were transferred to a 96-well plate, and the recombination in the cell supernatant was detected by dot-blot Protein expression level, select high-expressing cell clones into suspension culture.
[0039] The host cells are preferably mammalian cells, and Chinese hamster ovary (CHO) cells are used in this example, and human embryonic kidney 293 cells,...
Embodiment 3
[0041] Select cell lines with better expression levels and transfer them to 40ml shake flasks (Corning Company), select cell lines adapted to serum-free suspension culture, the medium used is SFM4 (Hyclone Company), and the culture conditions are 37°C, compare the cell growth curves , Recombinant protein expression level (quantitative ELISA detection, BD company, dy265), and finally determined the recombinant cell line 1F1G8 (expressing full-length recombinant human nerve growth factor (rhNGF)) and cell line 2F5 (expressing recombinant human nerve growth factor C-terminal deletion 3 amino acid mutant (rhNGF-D3)) as a recombinant cell line for further research.
[0042] The cell lines 1F1G8 and 2F5 were explored in a small-scale batch fed-batch cell culture process in a 10L WAVE bioreactor (Satorious Company), and the two cell lines maintained similar growth curves, such as Figure 4 As shown, but the expression level of rhNGF-D3 protein is about 10 times higher than that of rh...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com