Cefetamet pivoxil hydrochloride dispersible tablet and preparation method thereof
A technology for ceftazidime hydrochloride and dispersible tablets, applied in the field of medicine, can solve the problems of large volume of capsules, and achieve the effects of high bioavailability, short disintegration time and fast absorption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030]
[0031]
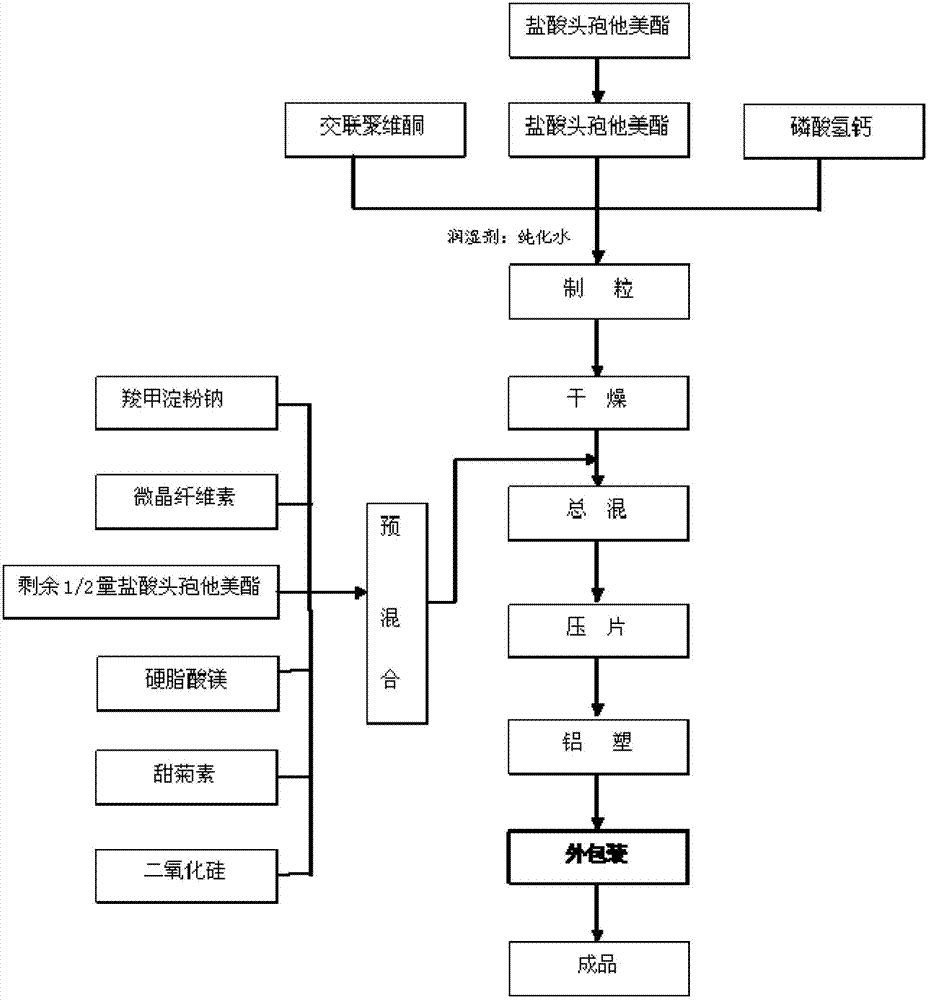
[0032] As shown in Figure 1, above-mentioned raw material prepares ceftazime pivoxil hydrochloride dispersible tablet according to the following method:
[0033] 1) Pretreatment of raw and auxiliary materials: ceftametexil hydrochloride, microcrystalline cellulose, crospovidone, calcium hydrogen phosphate, sodium carboxymethyl starch, silicon dioxide, magnesium stearate, stevioside all passed 60~ 80 mesh sieve;
[0034] 2) Granulation: Accurately weigh the sieved ceftazidime hydrochloride, crospovidone, and calcium hydrogen phosphate, pour them into a high-efficiency wet mixing granulator, set the stirring frequency to 40Hz, and start stirring and mixing for 3~5min After the mixing is completed, add purified water, set the chopping frequency to 45HZ, start chopping and granulating, and discharge;
[0035] 3) Drying: Spread the wet granules obtained from granulation evenly on the drying pan of the drying car, set the temperature at 50-65°C, and the tota...
Embodiment 2
[0042]
[0043] Above-mentioned raw material prepares ceftazidime hydrochloride dispersible tablet by the method described in embodiment 1.
Embodiment 3
[0045]
[0046] Above-mentioned raw material prepares ceftazidime hydrochloride dispersible tablet by the method described in embodiment 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com