Treatment of cardiac conditions
A technology for heart disease and ischemic heart disease, applied in the field of compound compound) as an inotropic agent for the treatment of cardiac dysfunction, which can solve the problem of unclear mechanism of action of gastric oxyntomodulin and inability to activate glucagon receptor And other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
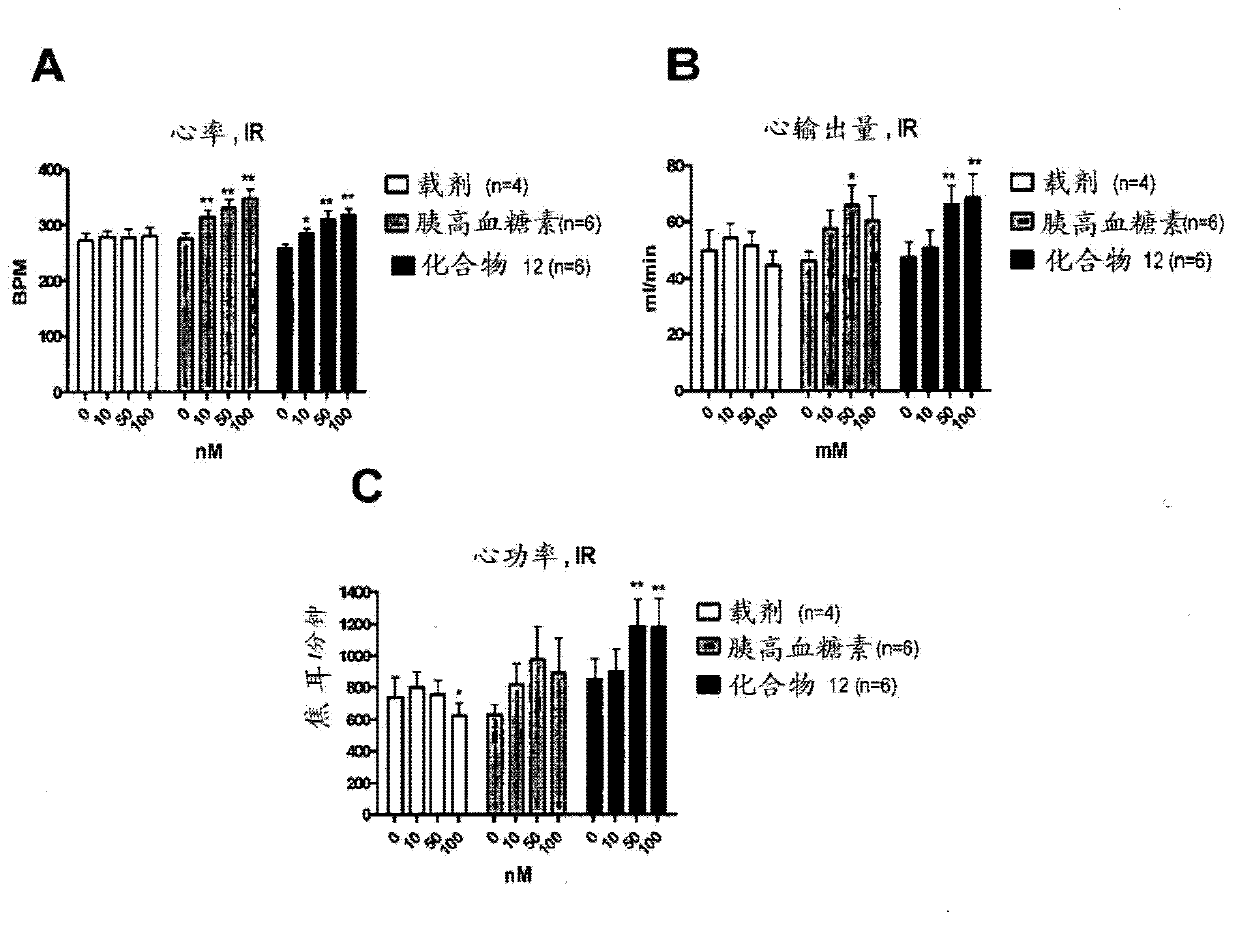
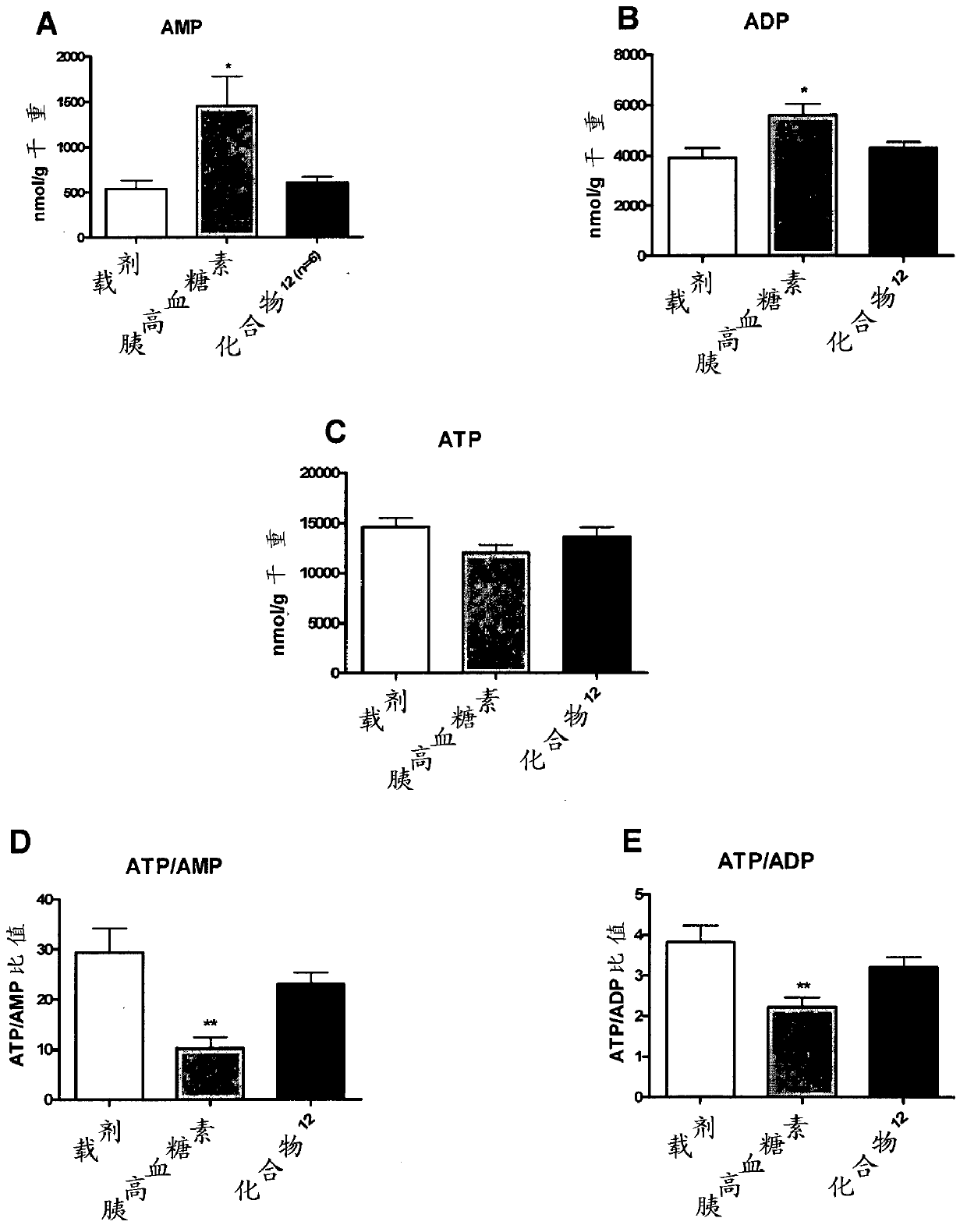
[1086] Example 1. Evaluation of inotropic effects in a working heart model
[1087] The inotropic compound glucagon and the glucagon-GLP-1 dual agonist (compound 12, with the sequence HSQGTFTSDYSKYLDRARADDFVAWLKST) were evaluated in isolated working hearts from control and insulin-resistant JCR:LA-cp rats. Role of cardiac function, metabolism and energy status (Lopaschuk, GD and Barr, RL. Measurements of fatty acid and carbohydrate metabolism in the isolated working rat heart. Molecular and Cellular Biochemistry. 1997; 172: 137-147). Isolated working hearts were perfused aerobically with Krebs-Henseleit solution (11 mM glucose, 2000 μU / ml insulin, 1.25 mM free Ca2+, 0.8 mM palmitate, and 3% BSA), and gradually added to the perfusion buffer during the perfusion. Increasing concentrations (10, 50 and 100 mM) of glucagon or compound 12. After perfusion, high-energy phosphate nucleotide concentrations were measured by high-performance liquid chromatography (HPLC) (Ally, A and Par...
Embodiment 2
[1089] Example 2. Determination of Potency on GLP-1 and Glucagon Receptors
[1090] HEK293 cells expressing human glucagon receptor or human GlP-1R (see above for details) were seeded at 40,000 cells / well into 96-well microtiter plates coated with 0.01% poly-L-lysine , and grown in cultures in 100 μl of growth medium for 1 day. On the day of analysis, the growth medium was discarded and the cells were washed once with 200 μl Tyrode's buffer. Cells were incubated for 15 minutes at 37°C in 100 µl of Tyrode's buffer containing increasing concentrations of the test peptide, 100 µM IBMX and 6 mM glucose. The reaction was stopped by adding 25 μl of 0.5M HCl and incubated on ice for 60 minutes. Using from Perkin-Elmer The cAMP kit assesses cAMP levels. Estimation of EC by computer-aided curve fitting 50 value.
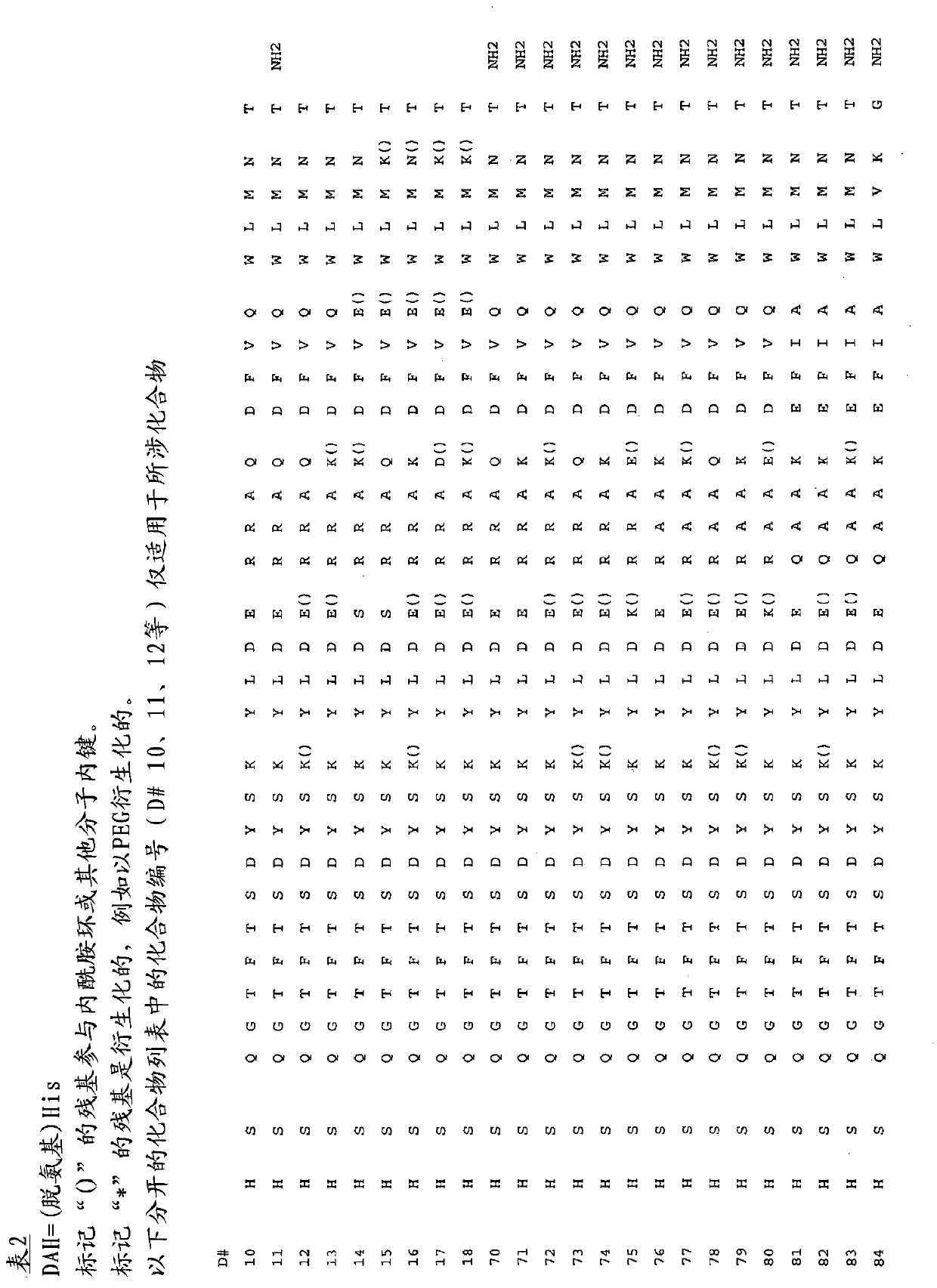
[1091] Table 1 shows sample compounds EC 50 value result.
[1092]
[1093]
[1094] Brackets () indicate intramolecular lactam rings.
Embodiment 3
[1095] Example 3. Evaluation of inotropic effects in anesthetized rats
[1096] The effects of inotropic Compound 1 and a glucagon-GLP-1 dual agonist (Compound 12) on cardiac function and heart rate were tested in anesthetized male Sprague-Dawley rats (Taconic) weighing approximately 300-400 g.
[1097] Rats were exposed to 1:2 N 2 O:O 2 in 5% isoflurane until anesthesia is confirmed. Body temperature was kept constant (37.5±0.5°C), animals were artificially ventilated by endotracheal intubation, and anesthesia was maintained.
[1098] The left femoral vein was catheterized for drug administration, and a pressure-volume catheter was inserted through the right carotid artery into the left ventricle. After installing the instrument, use pure O during the experiment 2 Delivery of isoflurane. After 20 minutes of stabilization, baseline data were recorded for 15 minutes while vehicle was being infused (at 7 L / min). Subsequently, the compound is infused. A 2.5 nmol / kg / min dos...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com