Method for preparing alpha, beta-unsaturated carbonyl compounds
A carbonyl compound, unsaturated technology, applied in the alpha field, can solve problems such as limited application, and achieve the effects of simple operation, high yield and short synthesis route
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
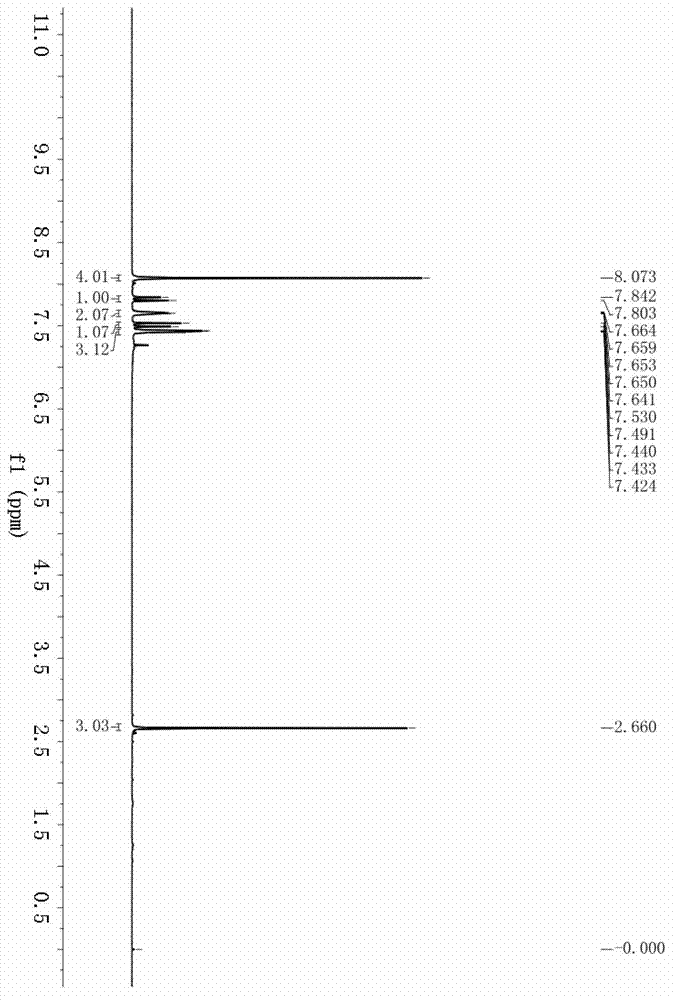
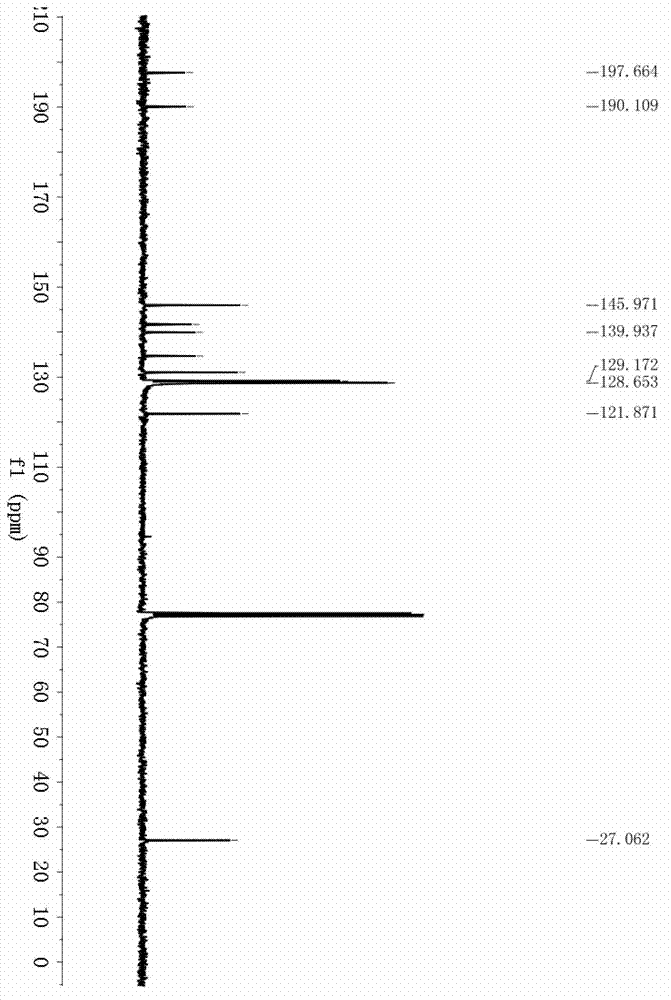
[0036] Example 1: Synthesis of (E)-1-(4-acetylphenyl)-3-phenylprop-2-en-1-one (3a) Accurately weigh 4-bromoacetophenone (99.5mg, 0.5mmol), hydrogen Potassium oxide (84.2mg, 1.5mmol), palladium chloride (4.4mg, 0.025mmol), and triphenylphosphine (26.2mg, 0.1mmol) were successively added to a 25mL autoclave, and after three times of nitrogen replacement, the Under nitrogen protection, 3 mL of tetrahydrofuran and phenylacetylene (102.1 mg, 1.0 mmol) were added. Fill the autoclave with CO to a pressure of 30atm, and stir at 60°C for 48h. After the reaction, add 10 mL of water to the reaction solution, extract 3 times with 3×10 mL of ethyl acetate, combine the organic phases, dry with anhydrous sodium sulfate for 1 h, remove the solvent under reduced pressure, and use petroleum ether / ethyl acetate as the eluent Reagent, silica gel column separation, (E)-1-(4-acetylphenyl)-3-phenylprop-2-en-1-one yield is 84%. 1 H NMR (400MHz, CDCl 3 )δ8.07(s,4H),7.82(d,J=15.6Hz,1H),7.67–7.64(m,2...
Embodiment 2
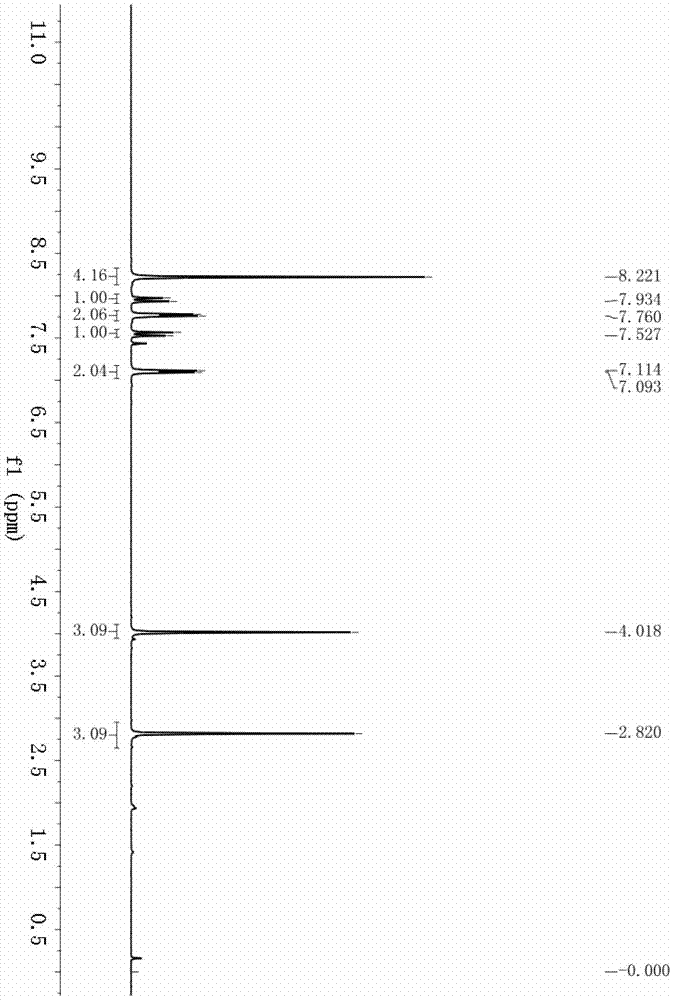
[0037] Example 2: Synthesis of (E)-1-(4-acetylphenyl)-3-(4-methoxyphenyl)prop-2-en-1-one (3b)
[0038] Accurately weigh 4-bromoacetophenone (99.5mg, 0.5mmol), sodium carbonate (159.0mg, 1.5mmol), palladium chloride (8.9mg, 0.05mmol), 1,4-bis(diphenylphosphino) Butane (42.6mg, 0.1mmol) was successively added into a 25mL autoclave. After three times of nitrogen replacement, 3mL of tetrahydrofuran and 4-methoxyphenylacetylene (132.2mg, 1.0mmol) were added under nitrogen protection. Fill the autoclave with CO until the pressure is 20atm, check whether there is air leakage after sealing, and stir at 150°C for 48h. After the reaction, add 10 mL of water to the reaction solution, extract 3 times with 3×10 mL of ethyl acetate, combine the organic phases, dry with anhydrous sodium sulfate for 1 h, remove the solvent under reduced pressure, and use petroleum ether / ethyl acetate as the eluent Reagent, silica gel column separation, (E)-1-(4-acetylphenyl)-3-(4-methoxyphenyl)prop-2-en-1-on...
Embodiment 3
[0039] Example 3: Synthesis of (E)-1-(4-acetylphenyl)-3-(p-tolyl)prop-2-en-1-one (3c) Accurately weigh 4-bromoacetophenone (99.5 mg, 0.5mmol), bis(triphenylphosphine)palladium dichloride (17.5mg, 0.05mmol), 1,5-bis(diphenylphosphine)pentane (44.0mg, 0.1mmol), and added to 25mL of In the autoclave, after three times of nitrogen replacement, 3 mL of anhydrous dimethyl sulfoxide, 4-methylphenylacetylene (174.2 mg, 1.5 mmol), and triethylamine (202.4 mg, 2.0 mmol) were added under nitrogen protection. Fill the autoclave with CO until the pressure is 20atm, check for air leakage after sealing, and stir at 100°C for 48h. After the reaction, add 10 mL of water to the reaction solution, extract 3 times with 3×10 mL of ethyl acetate, combine the organic phases, dry with anhydrous sodium sulfate for 1 h, remove the solvent under reduced pressure, and use petroleum ether / ethyl acetate as the eluent Reagent, silica gel column separation, (E)-1-(4-acetylphenyl)-3-(p-tolyl)prop-2-en-1-one ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com