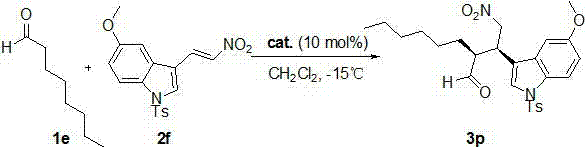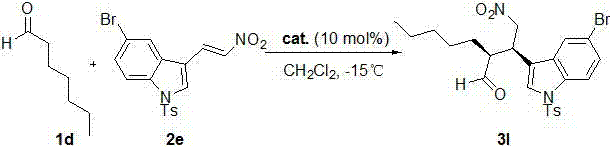Preparation method of chiral Beta-indolyl-Gamma-aldehyde group nitro alkanes
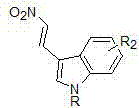
An aldehyde-nitroalkane and indolyl technology, which is applied in the field of asymmetric catalysis, can solve the problems of unreported and limited indole-derived nitroalkenes, and achieves simple and easy-to-obtain substrate raw materials, high compatibility, and usefulness. wide range of effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027]
[0028] (S)-diphenylprolinol trimethylsilyl ether (6.51 mg, 0.02 mmol) and 2a (37.6 mg, 0.2 mmol) were successively charged into the reaction flask, 1 mL of dichloromethane was added, and stirred at room temperature for 10 min , then added 1a (72 mg, 1 mmol), and the reaction was monitored by thin-layer chromatography until the reaction was completed. After 40 hours, the solvent was removed, and the crude product was subjected to simple column chromatography (eluent: ethyl acetate:petroleum ether=1:5 ) to obtain the target product, light yellow oil 3a (50.0 mg), with a yield of 96%.
[0029] The product was analyzed and the results were as follows: 96% yield, 98% ee, dr = 81 / 19. HPLC: Chiralcel OD-H, hexane / i-PrOH = 80:20, flow rate: 1.0 mL min -1 , λ = 210 nm, t major = 25.731 min, t minor = 20.987 min; [α] D 25 = -2.08 (c 2.16, CH 3 COCH 3 ); 1 H NMR (400 MHz, CDCl 3 ): δ 9.70 (d, J = 2.4 Hz, 1H), 8.29 (s, 1H), 7.55 (d, J = 8.0 Hz, 1H), 7.36-7.29 (m, 1H...
Embodiment 2
[0031]
[0032] (S)-Diphenylprolinol trimethylsilyl ether (6.5 mg, 0.02 mmol) and 2b (40.5 mg, 0.2 mmol) were successively charged into the reaction flask, 1 mL of dichloromethane was added, and stirred at room temperature for 10 min , then added 1a (72 mg, 1 mmol), and the reaction was monitored by thin-layer chromatography until the reaction was completed. After 72 hours, the solvent was removed, and the crude product was subjected to simple column chromatography (eluent: ethyl acetate:petroleum ether=1:5 ) to obtain the target product, light yellow oil 3b (43.9 mg), with a yield of 80%.
[0033] The product was analyzed and the results were as follows: 80% yield, 96% ee, dr = 93 / 7. HPLC: Chiralcel OD-H, hexane / i-PrOH = 80:20, flow rate: 1.0 mL min -1 , λ = 210 nm, t major = 27.079 min, t minor = 20.001 min; [α] D 25 = -6.30 (c 1.84, CH 3 COCH 3 ); 1 H NMR (400 MHz, CDCl 3 ): δ 9.72 (d, J = 2.4 Hz, 1H), 7.55 (d, J = 8.0 Hz, 1H), 7.31-7.22 (m, 2H), 7.17-7.11 (m,...
Embodiment 3
[0035]
[0036] (S)-diphenylprolinol trimethylsilyl ether (6.5 mg, 0.02 mmol) and 2c (65.7 mg, 0.2 mmol) were successively charged into the reaction flask, 1 mL of dichloromethane was added, and stirred at room temperature for 10 min , then added 1a (72 mg, 1 mmol), and the reaction was monitored by thin-layer chromatography until the reaction was completed. After 0.5 h, the solvent was removed, and the crude product was subjected to simple column chromatography (eluent: ethyl acetate:petroleum ether=1: 5) The target product, light yellow oil 3c (73.7 mg), was obtained with a yield of 92%.
[0037] The product was analyzed and the results were as follows: 92% yield, 99% ee, dr = 91 / 9. HPLC: Chiralcel OD-H, hexane / i-PrOH (80:20) , flow rate: 1.0 mL·min -1 , λ = 254 nm, t major = 41.177 min, t minor = 34.343 min; [α] D 25 = -3.52 (c 1.90, CH 3 COCH 3 ); 1 H NMR (400 MHz, CDCl 3 ): δ 9.72 (d, J = 1.6 Hz, 1H), 7.98 (d, J = 8.4 Hz, 1H), 7.79 (d, J = 7.6 Hz, 2H), 7.56-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com