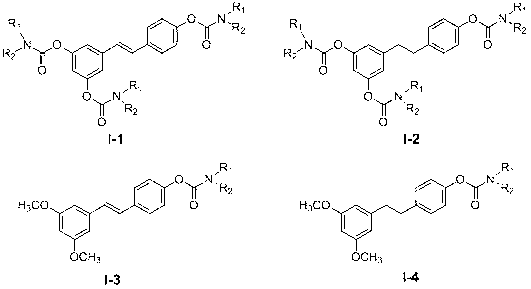
Carbamate compounds, preparation method and application thereof
A technology of carbamates and compounds, applied in the field of carbamates, their preparation and application, can solve the problems of multiple toxic and side effects, single action target, poor long-term curative effect of AD patients, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] ( E )- N,N - Preparation of 4-(3,5-dimethoxystyryl)phenyl dimethylcarbamate (I-3-1)
[0030] Add pterostilbene (2.0 mmol), K 2 CO 3 (3.0 mmol) and acetonitrile (20 ml), stir well and add N,N -Dimethylcarbamoyl chloride (3.0 mmol), reacted with stirring at room temperature for 15 hours (the reaction progress was followed by TLC); Dehydration: petroleum ether / ethyl acetate=5:1v / v) to obtain a white powder solid with a yield of 84.2%, mp: 124~125°C, HR-TOFMS (+Q) m / z :328.1557 ([C 19 h 21 NO 4 +H] + Calculated: 328.1549).
Embodiment 2
[0032] ( E )- N -methyl- N - Preparation of 4-(3,5-dimethoxystyryl)phenyl ethyl carbamate (I-3-2)
[0033] Operation process is the same as embodiment 1, just acetonitrile is replaced with chloroform, K 2 CO 3 Replaced with triethylamine, N,N -Dimethylcarbamoyl chloride N -methyl- N -Ethylcarbamoyl chloride was substituted to give a white powder solid with a yield of 79.3%, mp: 66.6~67.6℃, HR-TOFMS (+Q) m / z :342.1701 ([C 20 h 23 NO 4 +H] + Calculated: 342.1705).
Embodiment 3
[0035] ( E )- N,N - Preparation of 4-(3,5-dimethoxystyryl)phenyl diethylcarbamate (I-3-3)
[0036] Operation process is the same as embodiment 1, just will N,N -Dimethylcarbamoyl chloride N,N -Diethylcarbamoyl chloride was substituted to obtain a white powder solid with a yield of 82.7%, mp: 53.5~54.5°C, HR-TOFMS (+Q) m / z :356.1855 ([C 21 h 25 NO 4 +H] + Calculated value: 356.1862).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com