Preparation method and application of N, N-dimethyl-3, 3, 5-trimethyl cyclohexylamine
A technology of trimethylcyclohexylamine and dimethyl, applied in the field of N, to achieve the effect of simple production process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] By-product 3,3,5-trimethylcyclohexylamine in the manufacture of IPDA:
[0057] The reaction is carried out on a fixed-bed reaction equipment, which is composed of two-stage trickle-bed reactors. The first stage is filled with 100ml of zeolite, and the second stage is filled with 100ml of cobalt as the active component and diatomaceous earth as the carrier. hydrogenation catalyst, the reaction raw materials pass through the first reactor and the second reactor sequentially from top to bottom. The hydrogenation catalyst was reduced with pure hydrogen at 400°C for 12 hours before running the test. The controlled temperature of the first-stage reactor is 50°C, and the controlled temperature of the second-stage reactor is 90°C. The reaction pressure is 20MPa. The feed rate of IPN is 80g / h, the feed rate of ammonia is 137g / h, and the feed rate of hydrogen is 110 standard L / h. When the device runs for 100h, the reaction discharge is analyzed by gas chromatography, wherein is...
Embodiment 2
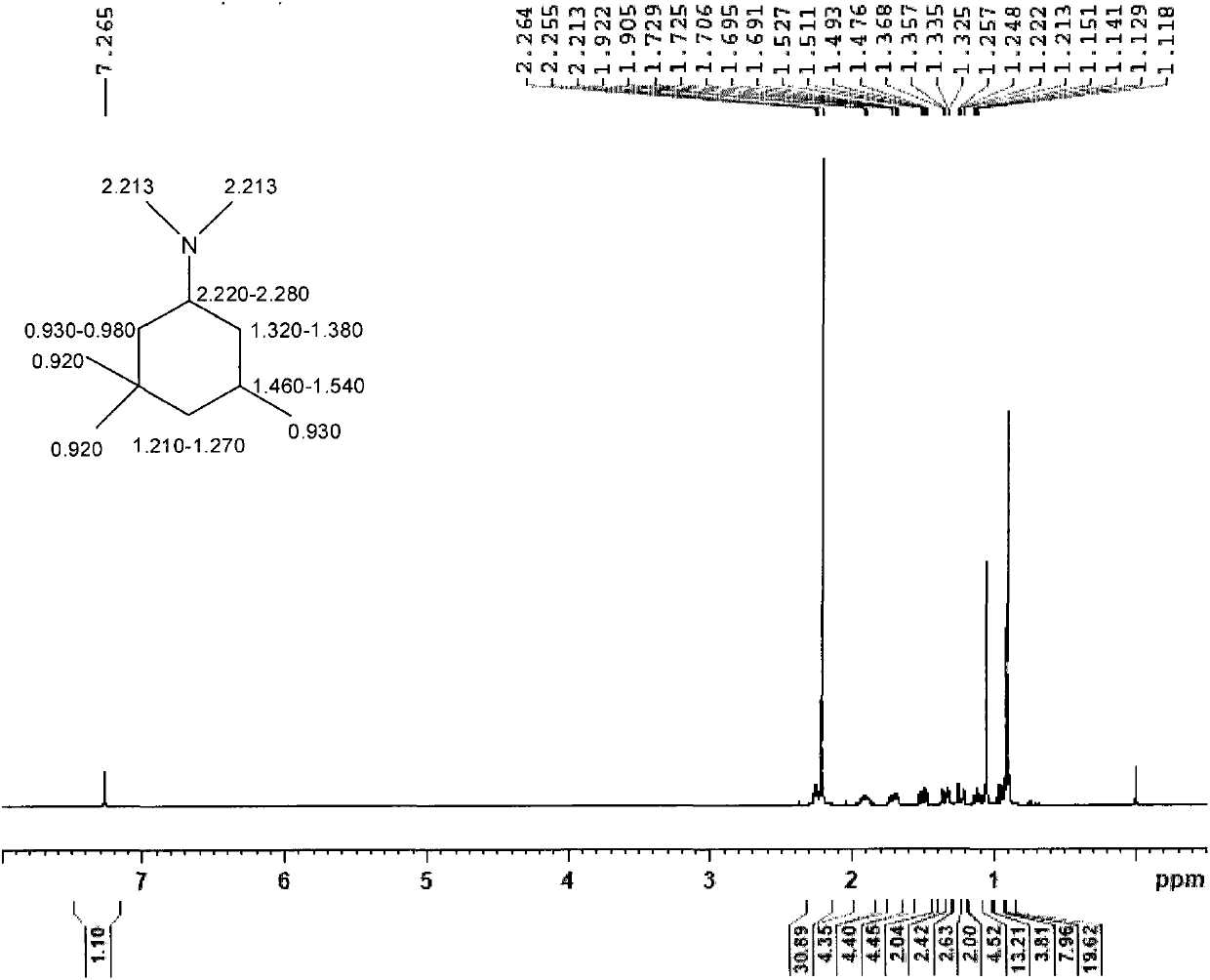
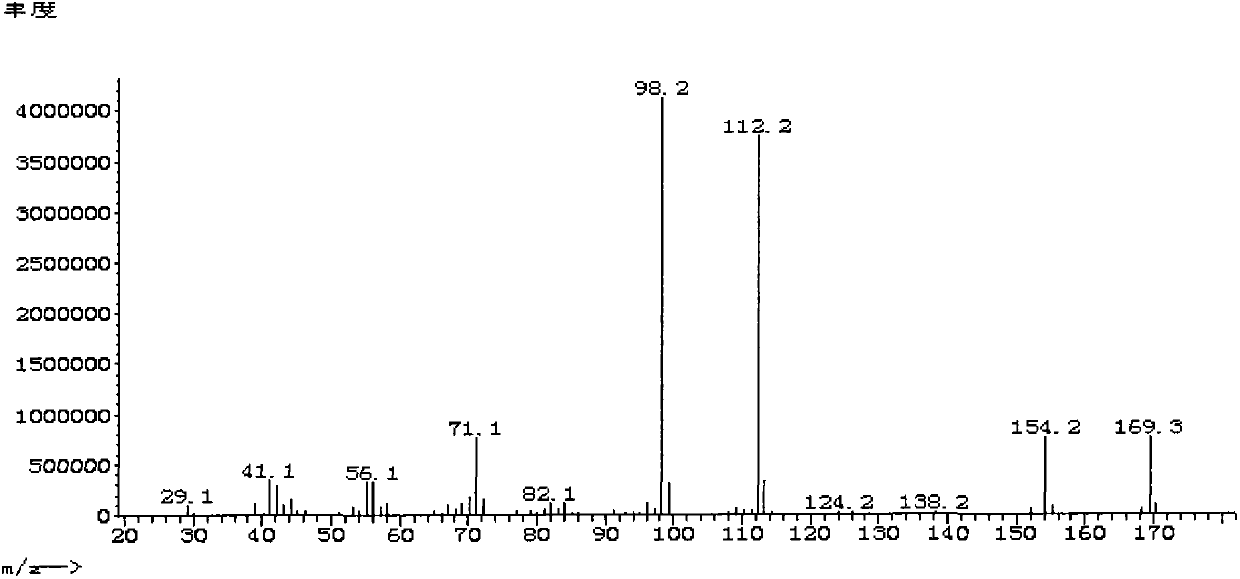
[0063] Embodiment 2: in the autoclave of 1L, add 140g 3,3,5-trimethylcyclohexylamine (the obtaining route of 3,3,5-trimethylcyclohexylamine is the same as embodiment 1), the content of 324g is 37% formaldehyde in water. 0.7 g of a common commercially available Pd / C catalyst with a Pd content of 5% was added. Seal the reaction kettle, replace the air with nitrogen 3 times, and replace the nitrogen with hydrogen 3 times. Turn on stirring to 600 rpm, raise the temperature in the reactor to 50° C., and increase the pressure in the reactor to 2 MPa. React until the system no longer absorbs hydrogen, and the reaction time is about 3 hours. After the reaction is finished, the reactant and the catalyst are filtered, and the reaction product is divided into a water phase and an oil phase. The oily and aqueous phases were separated using a pear-shaped separatory funnel. The oil phase was taken for gas chromatography analysis, and the content of N,N-dimethyl-3,3,5-trimethylcyclohexyl...
Embodiment 3
[0064] Embodiment 3: in the autoclave of 1L, add 140g 3,3,5-trimethylcyclohexylamine (the obtaining route of 3,3,5-trimethylcyclohexylamine is the same as embodiment 1), 243g content is 37% formaldehyde in water. 1.1 g of a common commercially available Ru / C catalyst with a Ru content of 5% was added. Seal the reaction kettle, replace the air with nitrogen 3 times, and replace the nitrogen with hydrogen 3 times. Turn on the stirring to 600 rpm, raise the temperature in the reactor to 70° C., and increase the pressure in the reactor to 3 MPa. React until the system no longer absorbs hydrogen, and the reaction time is about 3 hours. After the reaction is finished, the reactant and the catalyst are filtered, and the reaction product is divided into a water phase and an oil phase. Separate the oily and aqueous phases with a pear-shaped separatory funnel. The oil phase was taken for gas chromatography analysis, and the content of N,N-dimethyl-3,3,5-trimethylcyclohexylamine was ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com