Organic semiconductor material containing naphthalene, anthracene and dibenzothiophene sulfone unit, preparation method and application thereof
A technology of benzothiophene sulfone and organic semiconductor, which is applied in the field of organic semiconductor materials and its preparation, and can solve the problems of weak luminous intensity, low luminous rate, and poor carrier transport performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
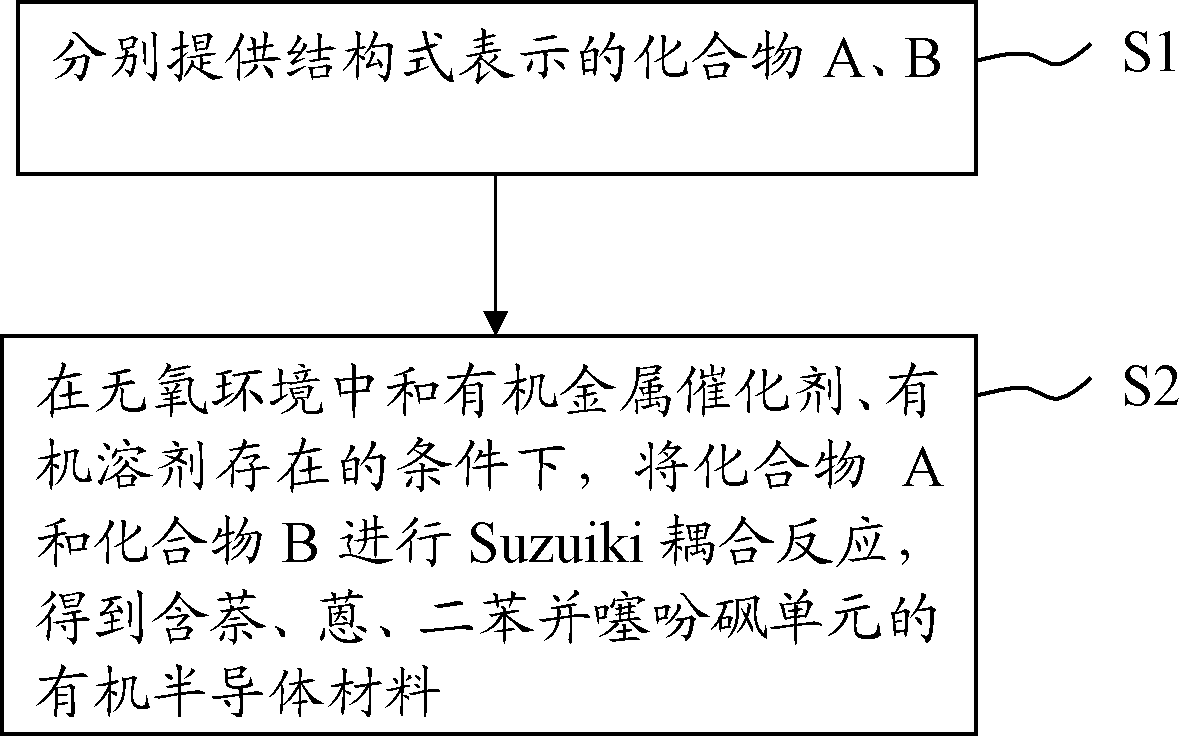
[0032] The embodiment of the present invention also provides a method for preparing the above-mentioned organic semiconductor material containing naphthalene, anthracene, and dibenzothiophene sulfone units. For the process flow of this method, please refer to figure 1 . The organic semiconductor material containing naphthalene, anthracene and dibenzothiophene sulfone units comprises the following steps:
[0033] S1: respectively provide compounds A and B represented by the following structural formulas, wherein R is a hydrogen atom, C 1 ~C 12 Alkyl or C 1 ~C 12 alkoxy,
[0034]
[0035] S2: In an oxygen-free environment and in the presence of organometallic catalysts and organic solvents, compounds A and B are subjected to a Suzuiki coupling reaction to obtain a naphthalene, anthracene, and dibenzothiophene sulfone represented by the general formula (I) The organic semiconductor material of unit; The Suzuiki coupling reaction formula of this step S2 can be expressed as...
Embodiment 1
[0048] The organic semiconductor material of the present embodiment naphthalene, anthracene, dibenzothiophene sulfone unit is the organic compound 2,7-two (10-(6-methoxy-naphthalene-2) containing naphthalene, anthracene, dibenzothiophene sulfone unit -base) anthracene-9-yl) dibenzothiophene sulfone (MODNAFSO) and preparation method thereof, its structural formula is as follows I 1 Shown:
[0049]
[0050] The preparation steps of above-mentioned polymer are as follows:
[0051] S11: respectively provide compounds A and B represented by the following structural formulas,
[0052]
[0053] Wherein, the specific preparation steps of compound A, namely 2,7-dibromodibenzothiophene sulfone are: dissolving 4 mmol of dibenzothiophene sulfone in 30 ml of concentrated H 2 SO 4 8.2mmol NBS was added at room temperature, and the reaction was stirred. After 24 hours of reaction, the reaction liquid was poured into water, filtered with suction, washed with water and methanol, and t...
Embodiment 2
[0061] The organic semiconductor material of the present embodiment naphthalene, anthracene, dibenzothiophene sulfone unit is the organic compound 2,7-bis(10-(6-tert-butoxy-naphthalene- 2-yl) anthracene-9-yl) dibenzothiophene sulfone (t-BuODNAFSO) and preparation method thereof, its structural formula is as follows I 2 Shown:
[0062]
[0063] The preparation steps of above-mentioned polymer are as follows:
[0064] S21: Provide compounds A and B represented by the following structural formulas respectively,
[0065]
[0066] Wherein, the method for obtaining compound A, that is, 2,7-dibromodibenzothiophene sulfone is the same as step S11 of Example 1.
[0067] S22: Preparation of 2,7-di(10-(6-tert-butoxy-naphthalene-2-yl)anthracen-9-yl)dibenzothiophene sulfone (t-BuODNAFSO), whose chemical reaction formula is as follows:
[0068]
[0069] The specific preparation process is: the above-mentioned compound A (2,7-dibromodibenzothiophene sulfone) 3mmol, compound B (10...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com