Novel stem cells, method for screening same, kit and application thereof
A technology of stem cells and kits, applied in the fields of new stem cells, their screening, kits and their uses, can solve problems such as low cell viability, affecting cell viability, improper operation, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0141] Example 1 Screening and Identification of New Liver Stem Cells
[0142] The liver tissue is obtained from rodents, humans or other mammals, normal liver tissue, biopsy or isolated liver tissue, and then the tissue is washed with PBS buffer solution for 2 to 3 times, and then the liver tissue is cut into pieces with ophthalmic scissors. 1mm 3 After adding trypsin (concentration: 0.1%~0.3%), digest at 37°C for 10mim~60min, add DMEM / F12K (Hyclone Company, USA) containing 10% fetal bovine serum equal to the volume of trypsin solution to stop Reaction, centrifuge at 800-1000g for 3mim-5min at low temperature, discard the supernatant, add low-temperature pre-cooled PBS containing 2% fetal bovine serum to wash the precipitate for 2-3 times, and filter with a sterile 100-400-mesh sieve to filter Take out the cell suspension, trypan blue staining live cells ≥ 96.7%.
[0143] Inoculate the isolated primary cell population in cell culture flasks or cell culture plates or culture...
Embodiment 2
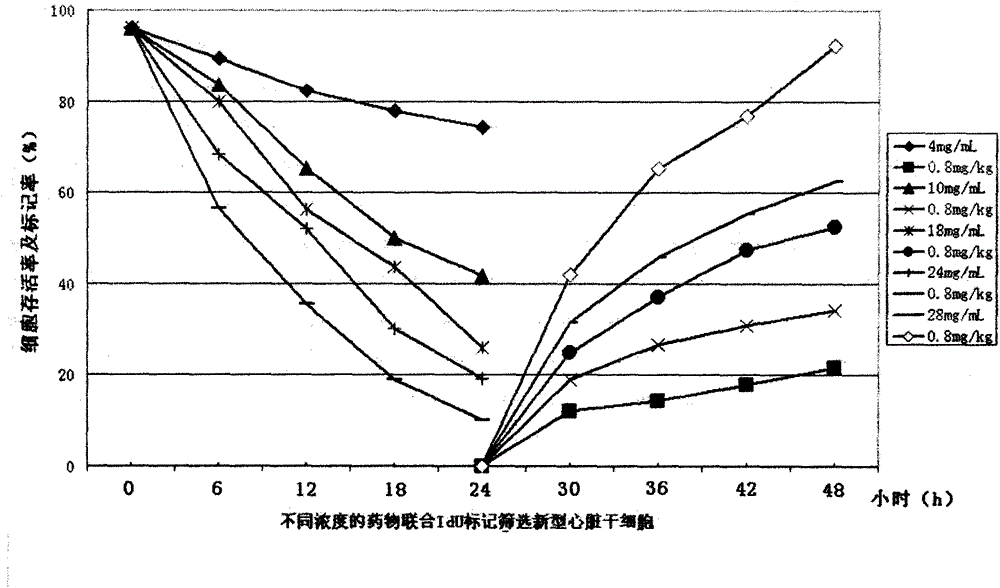
[0152] Example 2 Screening and Identification of New Cardiac Stem Cells
[0153] The heart tissue is obtained from the normal heart tissue of rodents, humans or other mammals, biopsy or isolated fresh heart tissue, and then the tissue is washed with PBS buffer for 2 to 3 times, and then the heart tissue is cut into pieces with ophthalmic scissors. About 0.5~1mm 3 Add collagenase (concentration: 2-10mg / mL) and digest at 37°C for 10mim-30min, add DMEM / F12K containing 10% fetal bovine serum (U.S. Hyclone Company) equal to the volume of collagenase solution to stop For reaction, centrifuge at 900 g for 3 min to 5 min at low temperature, discard the supernatant, add low-temperature pre-cooled PBS containing 2% fetal bovine serum to wash the precipitate for 2 to 3 times, and filter with a sterile 200 to 400 mesh screen to filter out the cell suspension. Solution, trypan blue staining live cells ≥ 95%.
[0154] The cell population was seeded in a 6-well cell culture plate at 37°C, ...
Embodiment 3
[0163] Example 3 Screening of novel cancer stem cells from liver cancer
[0164] Liver cancer tissues were obtained from rodents, humans or other mammals, biopsies, or fresh liver cancer tissues in vitro, and then washed with PBS buffer for 2 to 3 times, and then cut into pieces with ophthalmic scissors. about 1mm 3 Add trypsin (concentration: 0.1% ~ 0.3%) and collagenase (concentration: 1 ~ 10mg / mL) and digest at 37°C for 5mim ~ 60min, add the same volume of enzyme solution containing 10% DMEM / F12K (U.S. Hyclone Company) of fetal bovine serum was used to stop the reaction, and the cell suspension was gently blown 2-3 times, centrifuged at 800-1000g for 3mim-5min at low temperature, discarded the supernatant, and added low-temperature pre-cooled 2% fetal bovine The serum was washed with PBS for two to three times, filtered through a sterile 200-400-mesh sieve to filter out the cell suspension, and trypan blue stained viable cells > 96%.
[0165] Inoculate the isolated primar...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com