Pegylated celastrol and preparation method and application thereof
A technology of triptolide and pegylation, which is applied in the field of medicine, can solve the problems of no triptolide drug, high toxicity, and marketing, and achieves the effect of prolonging the elimination half-life, reducing the toxic and side effects, and reducing the frequency of administration. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Example 1: mPEG 10k - Preparation of NHCO-Celastrol
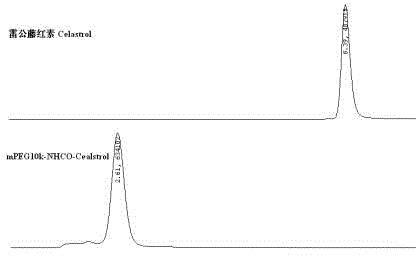
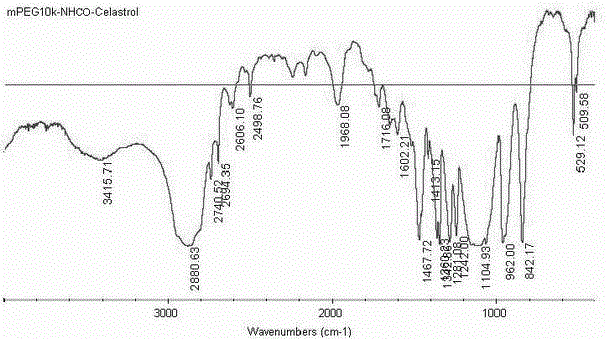
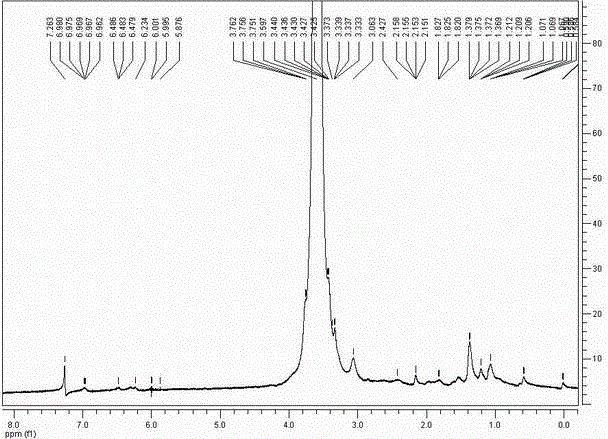
[0040] Tripterine 4.5 g (10 mmol) was added with DCC 2.06 g (10 mmol), DMAP 0.5 g (4 mmol), TEA 550 μg (5.45 mmol) and methoxyaminopolyethylene glycol 10 kDa (mPEG 10k -CH 2 CH 2 -NH 2 HCl) in 50 g (4.76 mmol) of anhydrous dichloromethane solution, react at room temperature, and monitor the reaction process by spotting on a silica gel plate. After the reaction is complete, filter the reaction solution, evaporate the filtrate to dryness, add deionized water, extract with dichloromethane, dry the organic phase with anhydrous sodium sulfate, concentrate, and precipitate with cold ether. Multiple washes to obtain mPEG 10k -NHCO-Celastrol product 52.13 g, is a red-yellow solid, and its modification rate was determined by HPLC-UV.
[0041] mPEG 10k -NHCO-Celastrol has a molecular weight of 10981.5 (MALDI-TOF-TOF).
Embodiment 2
[0042] Example 2: mPEG 10k - Preparation of NHCO-Celastrol
[0043] 0 ℃ filled with N 2 Under the conditions of chloroformate, 0.642 g (4.7 mmol) of isobutyl chloroformate was added to anhydrous dichloromethane solution containing 4.5 g (10 mmol) of tripterine (10 mmol) and 1.5 g (15.15 mmol) of NMP, and the reaction was carried out for 20 min, followed by the addition of mPEG pre-supplemented with TEA 550 μg (5.45 mmol) 10k -CH 2 CH 2 -NH 2 50 g (4.76 mmol) of HCl in dichloromethane solution, kept at 0 °C for 2 h, moved to room temperature to continue the reaction, and monitored the reaction progress by spotting on a silica gel plate. After the reaction is complete, filter the reaction solution, evaporate the filtrate to dryness, add deionized water, extract with dichloromethane, dry the organic phase with anhydrous sodium sulfate, concentrate, and precipitate with cold ether. Multiple washes to obtain mPEG 10k -NHCO-Celastrol product 51.65 g, is a reddish-yellow...
Embodiment 3
[0044] Example 3: mPEG 10k - Preparation of NHCO-Celastrol
[0045] Add 4.5 g (10 mmol) of tripterine into anhydrous dichloromethane solution containing 0.863 g (7.5 mmol) of NHS and 2.06 g (10 mmol) of DCC, react at 25°C, filter, evaporate the filtrate to dryness, and obtain Tripterygium wilfordii Ruby succinimidyl ester. In addition mPEG 10k -CH 2 CH 2 -NH 2 Dissolve 50 g (4.76 mmol) of HCl in anhydrous dichloromethane solution, adjust the pH to 9.0 with TEA, add the above-mentioned tripterine succinimide ester, react at 25 °C, and monitor the reaction process by spotting on a silica gel plate. After the reaction is complete, filter the reaction solution, evaporate the filtrate to dryness, add deionized water, extract with dichloromethane, dry the organic phase with anhydrous sodium sulfate, concentrate, and precipitate with cold ether. Multiple washes to obtain mPEG 10k -NHCO-Celastrol product 52.05 g, a reddish-yellow solid, its modification rate was determine...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com