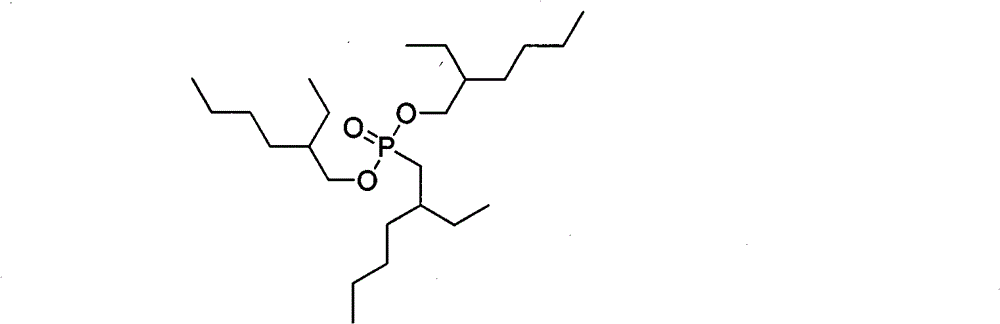
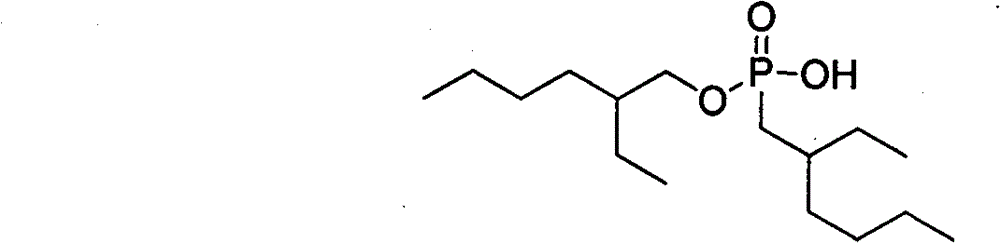
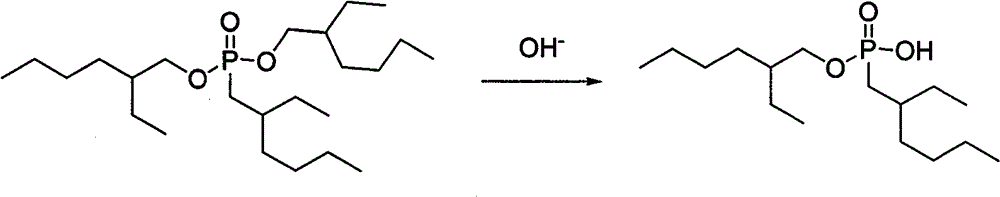
Method for preparing 0,0-di(2-ethylhexyl)-2-ethylhexyl phosphonate
A technology of hexylphosphonate and ethylhexyl, applied in the field of 0, can solve the problems of strong corrosiveness of raw materials, cumbersome processing process, difficult process control, etc., and achieve the effects of reducing reaction temperature, reducing reaction procedures and reducing production cost.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] The preparation method of 0,0-two (2-ethylhexyl)-2-ethylhexylphosphonate of the present invention, it comprises the steps:
[0035] A, 77 parts by weight of toluene, 20.2 parts by weight of isooctyl alcohol, and 15.2 parts by weight of dry triethylamine are placed in the reaction vessel, then the materials in the reaction vessel are stirred, and then the materials in the reaction vessel are heated up to 50°C or 55°C or 60°C or 65°C or 70°C or 75°C or 80°C, then add 6.85 parts by weight of phosphorus trichloride dropwise to the reaction vessel, the time for the addition is 80-100min, and the addition of trichloride After phosphorus removal, keep at 40°C or 45°C or 50°C or 55°C or 60°C or 65°C or 70°C or 75°C or 80°C or 85°C or 90°C for 1.5h-2h, then cool, filter, and the mother liquor is often The toluene was distilled under pressure to obtain the crude product, and the crude product was distilled under reduced pressure to obtain triisooctyl phosphite, and the yield of t...
Embodiment 2
[0040] The preparation method of 0,0-two (2-ethylhexyl)-2-ethylhexylphosphonate of the present invention, it comprises the steps:
[0041] (1) Synthesis of triisooctyl phosphite: add 70g toluene, 20.2g (0.155mol) isooctyl alcohol, and 15.2 g (0.15 mol) of dry triethylamine. Start stirring, and after heating up to 40-90° C., slowly add 6.85 g (0.05 mol) of phosphorus trichloride dropwise from a constant pressure dropping funnel. The molar ratio of isooctyl alcohol and phosphine trichloride is 3.1:1. After the addition, continue to react at this temperature for 1.5-2h, cool, filter, distill toluene from the mother liquor under normal pressure to obtain the crude product, and distill the crude product under reduced pressure to obtain sub Tri-isooctyl phosphate, yield 96%.
[0042](2) Synthesis of 0,0-di(2-ethylhexyl)-2-ethylhexylphosphonate: 0.78g (0.005mol, 0.125eqv) of bromoisoctane, triphosphite 16.7g (0.04mol, 1eqv) of isooctyl ester and 1.48g (0.004mol, 0.1eqv) of tetrabu...
Embodiment 3
[0044] The preparation method of 0,0-two (2-ethylhexyl)-2-ethylhexylphosphonate of the present invention, it comprises the steps:
[0045] (1) Synthesis of triisooctyl phosphite: add 70g toluene, 20.2g isooctyl alcohol, and 11.9g (0.15 mol) dry pyridine. Stirring was started, and after the temperature was raised to 55° C., 6.85 g (0.05 mol) of phosphorus trichloride was slowly added dropwise from a constant pressure dropping funnel. After the addition, continue to react at this temperature for 1.5 h, cool, filter, distill toluene from the mother liquor under atmospheric pressure to obtain a crude product, and distill the crude product under reduced pressure to obtain tri-isooctyl phosphite with a yield of 96.6%.
[0046] (2) Synthesis of 0,0-bis(2-ethylhexyl)-2-ethylhexylphosphonate: 0.78g (0.005mol) of bromoisoctane and triisooctyl phosphite were placed in a 100ml three-necked flask 16.7g (0.04mol), 1.29g (0.004mol) of tetrabutylammonium bromide, reacted overnight at a temp...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com