Composite medicine for targeted elimination of HIV/SIV
A drug and fusion protein technology, applied in the field of fusion proteins, can solve problems such as ineffectiveness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
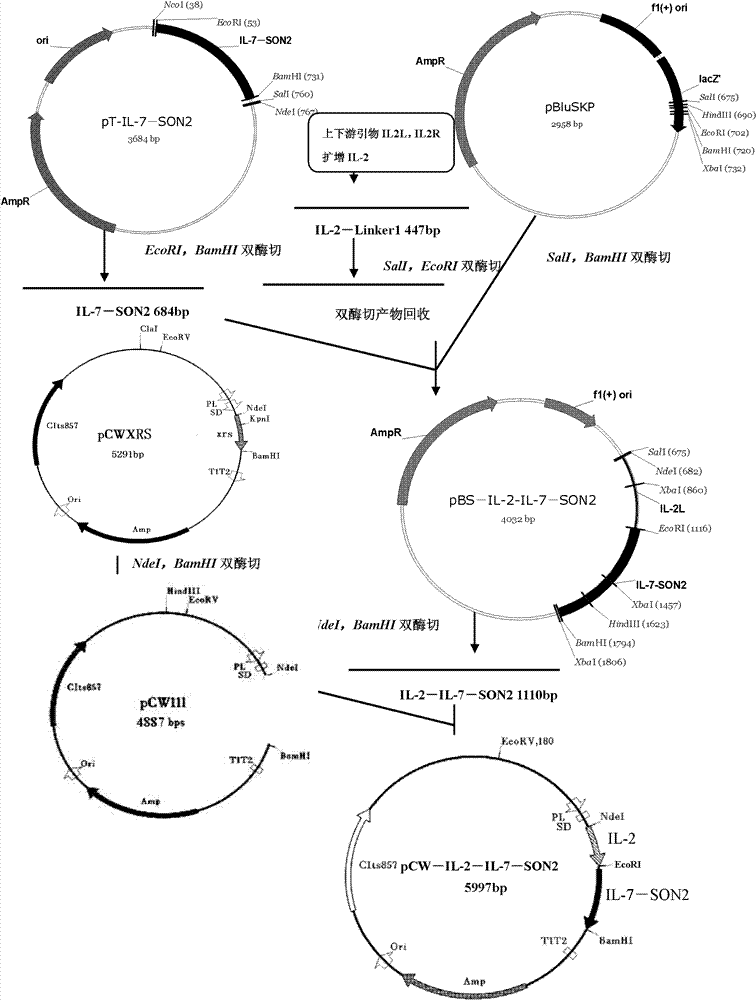
[0083] Example 1 Obtaining of Gene IL2 Fragment Encoding Recombination Targeting Fusion Protein
[0084] With the recombinant human IL2 gene containing C58S and C126S mutations (use PCR technology to change the DNA coding sequence of cysteine Cysteine at the 58th position of human IL2 into the coding sequence of serine Serine; change the cysteine Cysteine at the 126th position The DNA coding sequence is also changed to the coding sequence of serine Serine. In this way, through the expression of the recombinant plasmid, the derivative of IL2 is obtained, and its 58th and 126th positions are all serine. Such IL2 derivatives can only combine with its receptor The ability to inactivate IL2R without other biological activity.) as a template, the following primers were used to amplify the mutated IL2 gene fragment:
[0085] (1) Primer IL2L: gga ggt cga cca tat ggc acc tac ttc aag ttc, a total of 33mers, containing restriction enzymes SalI and NdeI cutting sites; (SEQ ID NO: ...
Embodiment 2
[0088] Example 2 Obtaining of Gene IL7 Fragment Encoding Recombination Targeting Fusion Protein
[0089] The IL7 gene used in the present invention comes from the human genome (see SEQ ID NO: 3 for its DNA coding sequence), and the codons therein are mainly encoded according to the frequency of codon usage preferred by eukaryotes. To express IL7 in prokaryotes requires Modify its codons. According to the characteristics of the codon usage frequency in the IL7 gene, the present invention uses the method of whole gene synthesis to transform the codon of IL7 so that it conforms to the codon preference of the host Escherichia coli, and the nucleotide sequence of the transformed IL7 is shown in SEQ ID NO: 4, see SEQ ID NO: 5 for the amino acid sequence. In addition, the coding sequence of Linker2 and the HindIII restriction site were fused to the 3' end of IL7 during synthesis, and placed in the vector pMD18-T. The primer sequences used in this embodiment are as follows:
[0090...
Embodiment 3
[0093] Example 3 Obtaining of Gene IL7-SON2 Fragment Encoding Recombination Targeting Fusion Protein
[0094] The present invention uses newIL7L containing restriction site EcoRI as the upstream primer (SEQ ID NO: 26), pSON1 (SEQ ID NO: 27), pSON2 (SEQ ID NO: 28), pSON3 (SEQ ID NO: 29), pSON4 (SEQ ID NO: 30), pSON5 (SEQ ID NO: 31) (including restriction site BamHI) as downstream primers, after 5 rounds of amplification, a fusion fragment containing IL7 coding sequence, SON2 coding sequence and Linker2 totaling 684bp was obtained. The results of agarose gel electrophoresis showed that the target product was obtained. Restriction sites EcoRI and BamHI were introduced into the upstream and downstream of the fusion fragment respectively. The fusion fragment was TA cloned into pGEM-T for sequencing. Sequencing results showed that the cloned fragment was correct, and the positive clone was named pT-IL7-SON2.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com