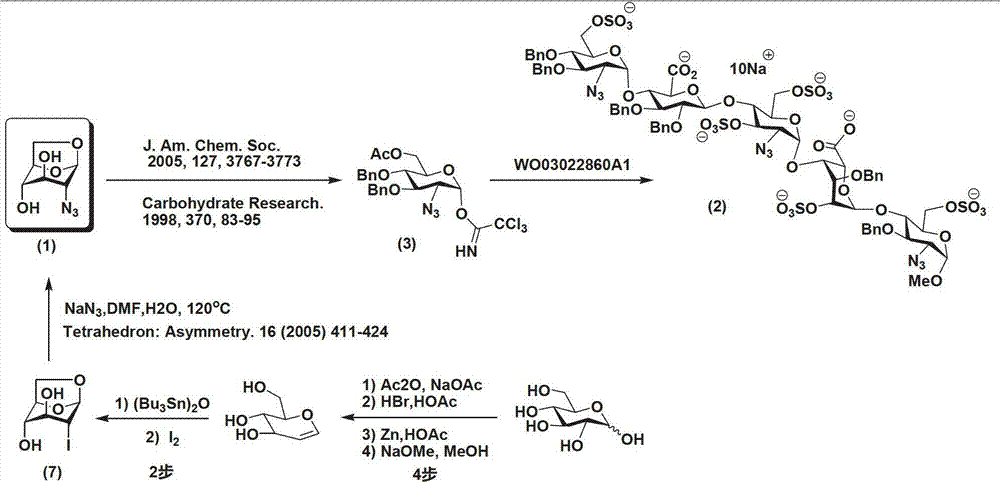
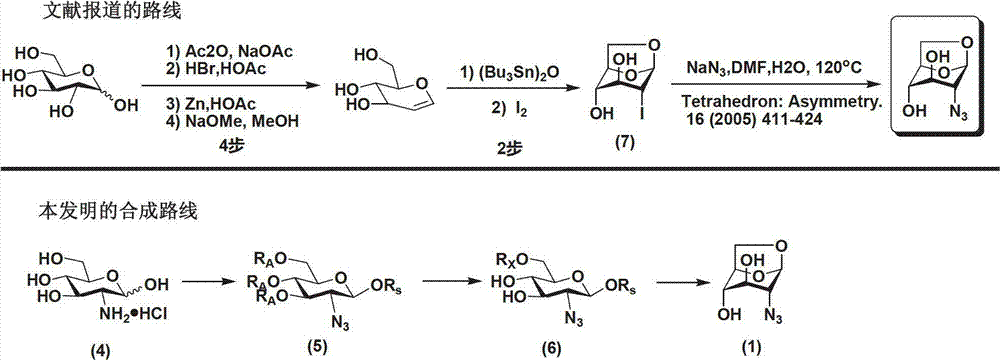
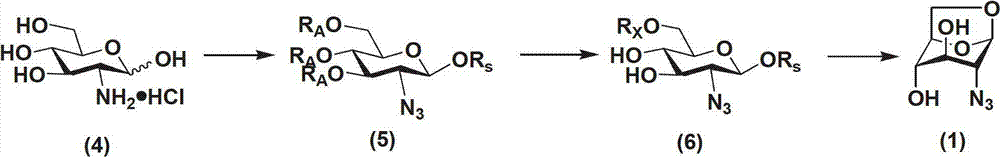Method for preparing 1,6-Anhydro-2-azido-2-deoxy-beta-D-glucopyranose
A technology of glucopyranose and sulfonyl azide hydrochloride, which is applied in the field of pharmaceutical synthesis technology, can solve problems such as unfavorable process amplification, unfavorable safety operation, high explosion risk, etc., and is conducive to process amplification to achieve large-scale production , simplification of post-processing operations, the effect of safety explosion risk
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Example 1 Preparation of 1,6-anhydro-2-azido-2-deoxy-β-D-glucopyranose
[0030]
[0031] Step 1: At room temperature, add 12.77g of 2-amino-2-deoxy-D-glucopyranose hydrochloride (4), 148mg of copper sulfate pentahydrate, and 22.1g of potassium carbonate to 300mL of methanol in turn, stir well; replacement. Cool to 0°C, add 14.9g imidazole-1-sulfonyl azide hydrochloride in batches, and stir evenly. Stirring was continued for 12 h at room temperature, and the reaction was complete. The solid was removed by suction filtration, evaporated to dryness, and carried twice with 200 mL of toluene to obtain a residue. The residue was dissolved in 400 mL of dichloromethane, stirred evenly, cooled to 0°C, 70 g of Dowex 1X8 resin was added, and 30 mL of acetic anhydride was slowly added dropwise while stirring. After dripping, rise to room temperature and continue to stir for ~12h, the reaction is complete. The resin was removed by filtration, and the solvent was evaporated to...
Embodiment 2
[0034] Example 2 Preparation of 1,6-anhydro-2-azido-2-deoxy-β-D-glucopyranose
[0035]
[0036] The first step, second step: same as embodiment 1
[0037] The third step: 10.3g 1-O-silyl-6-O-p-toluenesulfonyl-2-azido-2-deoxy-β-D-glucopyranose (6) was dissolved in 150mL tetrahydrofuran, nitrogen protection, cold to 0°C. Add 1.6mL of acetic acid and 12mL of 2M hydrogen fluoride pyridine complex successively. Stirring was continued at 0 °C for ~8 h, and the reaction was complete. The tetrahydrofuran was evaporated, and the residue was dissolved in 150 mL of ethanol. At room temperature, 30 g of Dowex1X8 resin was added, and stirring was continued at room temperature for ~24 hours after the addition. After the reaction was complete, the resin was removed by filtration, and the solvent was evaporated. Purified by column chromatography to obtain 3.3 g of 1,6-anhydro-2-azido-2-deoxy-β-D-glucopyranose (1), with a yield of 81%. 1 H NMR(500MHz,DMSO-6d):5.40(d,J=4.5Hz,1H),5.37(s,1...
Embodiment 3
[0038] Example 3 Preparation of 1,6-anhydro-2-azido-2-deoxy-β-D-glucopyranose
[0039]
[0040] Step 1: At room temperature, add 12.77g of 2-amino-2-deoxy-D-glucopyranose hydrochloride (4), 300mg of copper sulfate pentahydrate, and 21g of potassium carbonate to 400mL of methanol in turn, stir well; replace with nitrogen . Cool to 0°C, add 15.3g imidazole-1-sulfonyl azide hydrochloride in batches, and stir evenly. Stirring was continued for 18 h at room temperature, and the reaction was complete. The solid was removed by suction filtration, evaporated to dryness, and carried twice with 200 mL of toluene to obtain a residue. The residue was dissolved in 400 mL of dichloromethane, stirred evenly, cooled to 0°C, 80 g of Dowex 1X8 resin was added, and 50 mL of acetic anhydride was slowly added dropwise while stirring. After dripping, rise to room temperature and continue to stir for 18h, the reaction is complete. The resin was removed by filtration, and the solvent was evapo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com