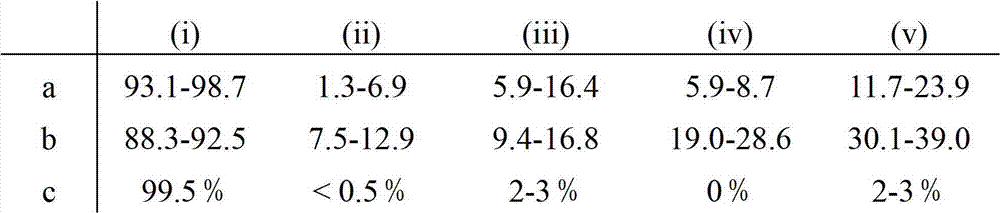
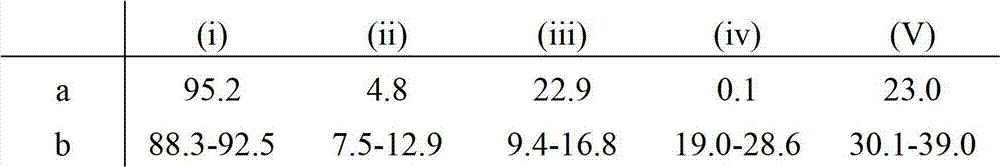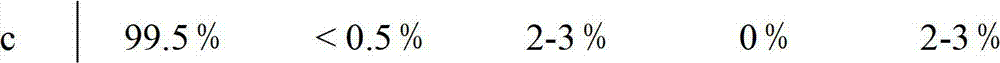Factor VII glycoforms
A factor and serum technology, applied in coagulation/fibrinolytic factors, blood diseases, genetic engineering, etc., can solve problems such as differences
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0119] Method: The present invention relates to a method for preparing a preparation comprising any one of the above-mentioned (i)-(vi) glycosyl patterns, and in a further embodiment, the present invention relates to making coagulation factor VII and coagulation factor VII-related polypeptides A method for optimizing the glycotype distribution of . These methods include the following steps:
[0120] (a) cultivating cells capable of expressing coagulation factor VII or coagulation factor VII-related polypeptides under a first set of predetermined culture conditions;
[0121] (b) recovering coagulation factor VII or coagulation factor VII-related polypeptides from the above culture to obtain preparations comprising these polypeptides; and
[0122] (c) analyzing the structure of the oligosaccharides linked to these polypeptides to determine the glycosylation pattern.
[0123] The method also includes:
[0124] (d1) changing the culture conditions of step (a) to obtain a second...
Embodiment 1
[0161] Example 1: Preparation and Analysis of Factor VII Preparations with Altered Glycotype Patterns
[0162] Factor VII preparations with altered glycoform patterns were prepared using the following experiment.
[0163] I. Preparation: A BHK cell line transformed with a plasmid encoding Factor VII was adapted to grow in suspension culture in the absence of serum. The cells were continuously passaged in a spinner culture, and the volume gradually increased due to the addition of new medium as the number of cells increased.
[0164] Finally, 6L of the seed culture was inoculated into a 100-liter preparative reactor containing a large-pore Cytopore 1 carrier (Pharmacia), on which the suspension cells were thus immobilized. Cultures were maintained at 36°C, pH 6.7-6.9 and 50% DO. As the number of cells increases, the volume in the preparative reactor gradually increases due to the addition of new medium. When the cell density reaches about 2×10 6 Cells / ml, start to enter t...
Embodiment 2
[0179] Example 2: Analysis of Factor VII Preparations with Altered Glycotype Patterns
[0180] Factor VII was prepared as described in Example 1 except that Factor VII was harvested from the 500-l culture. Glycotype analysis was performed as described in Example 1. Three different formulations (A, B, and C) were analyzed and compared to a reference formulation (D).
[0181]Bioavailability was determined in a canine model as follows: 12 Beagle dogs were divided into 4 groups for a four-limb crossover study. Each animal was dosed with test formulation A, B, or C, or reference formulation D, at a dose of approximately 90 μg / kg in N-glycylglycine buffer (pH 5.5) containing Sodium (2.92mg / ml), Calcium Chloride Dihydrate (1.47mg / ml), Mannitol (30mg / ml) and Polysorbate 80. Blood samples were taken at 10, 30, and 60 minutes and at 2, 3, 4, 6, and 8 hours post-dose. Plasma was obtained from these blood samples and factor VII was quantified by ELISA.
[0182] The bioavailability ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Titer | aaaaa | aaaaa |
| Titer | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com