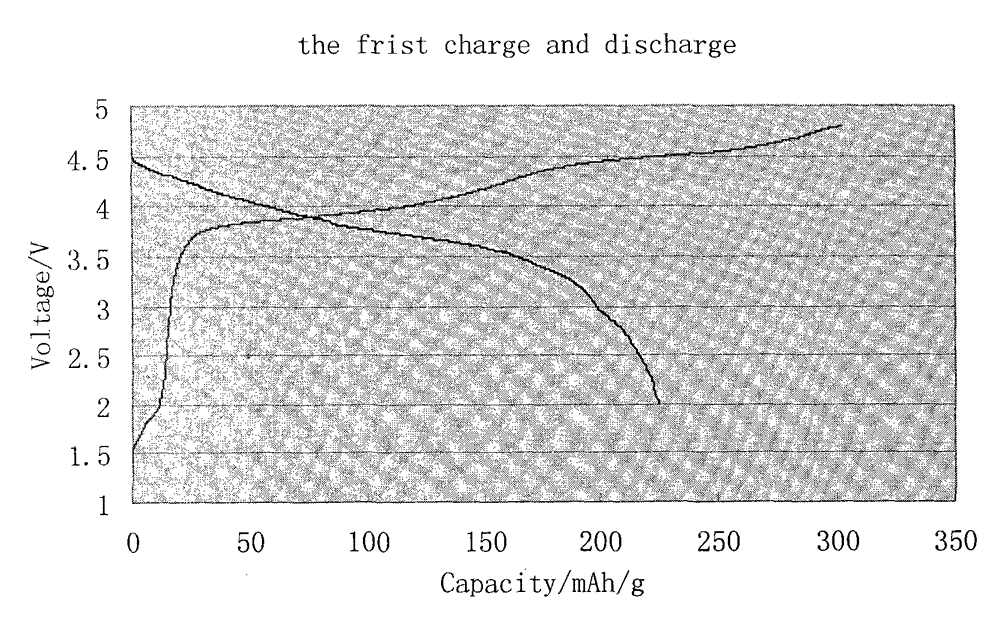
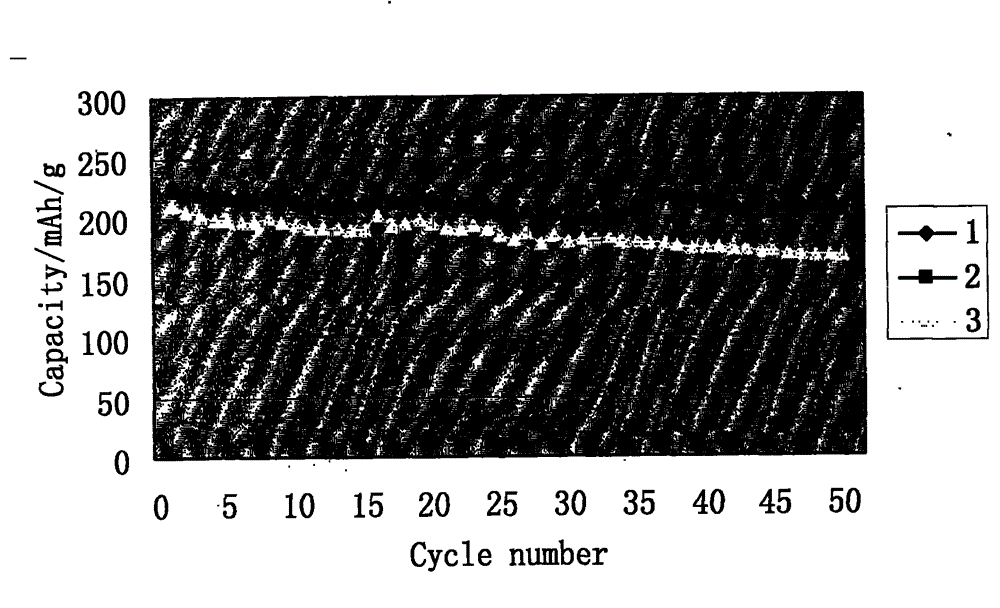
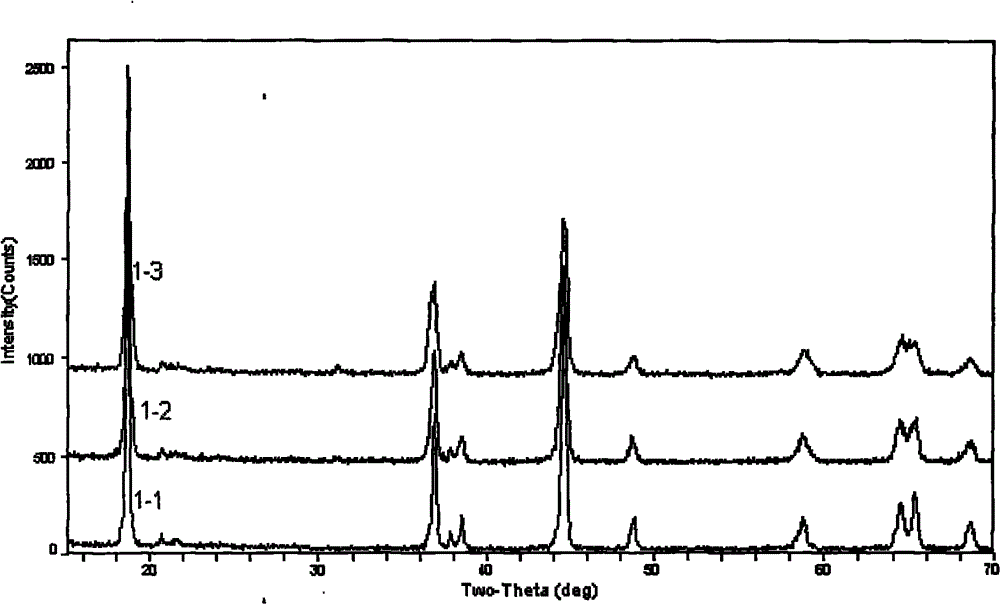
Coated lithium-rich manganese base material and preparation method thereof
A lithium-rich manganese-based, coated technology, applied in the direction of electrical components, battery electrodes, circuits, etc., can solve the problems of instability, difficult preparation, high price, etc., and achieve high charge and discharge voltage, good cycle performance, and low cost effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Production process steps:
[0036] a. With the molar ratio of nickel, cobalt and manganese metal ions as 0.233: 0.233: 0.533, nickel sulfate, cobalt sulfate and manganese sulfate are dissolved in deionized water, and the uniform concentration of nickel, cobalt and manganese ions is 1mol / L. Solution, prepare a mixed solution of 2mol / L sodium hydroxide and 3mol / L ammonia water, pump the mixed solution metal salt, ammonia water and sodium hydroxide solution into the reactor at the same time for coprecipitation reaction, and control the particle size of coprecipitation D. 50 =9μm; then nickel sulfate and manganese sulfate are dissolved in deionized water with the molar ratio of nickel and manganese metal ions being 0.5:1.5 to form a uniform mixed solution with a total concentration of nickel and manganese ions of 0.1mol / L, and the mixed The solution metal salt, ammonia water and sodium hydroxide are pumped into the reaction kettle at the same time, and the co-precipitatio...
Embodiment 2
[0041] Production process steps:
[0042] a. With the molar ratio of nickel, cobalt and manganese metal ions as 0.256: 0.112: 0.632, nickel sulfate, cobalt sulfate and manganese sulfate are dissolved in deionized water, and the uniform concentration of nickel, cobalt and manganese ions is 1mol / L. Solution, prepare a mixed solution of 2mol / L sodium hydroxide and 3mol / L ammonia water, pump the mixed solution metal salt, ammonia water and sodium hydroxide solution into the reactor at the same time for coprecipitation reaction, and control the particle size of coprecipitation D. 50 = 11 μm; then nickel sulfate and manganese sulfate are dissolved in deionized water with the molar ratio of nickel and manganese metal ions as 1:1 to form a uniform mixed solution with a total concentration of nickel and manganese ions of 0.1mol / L. The solution metal salt, ammonia water and sodium hydroxide are pumped into the reaction kettle at the same time for co-precipitation reaction. The product...
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com