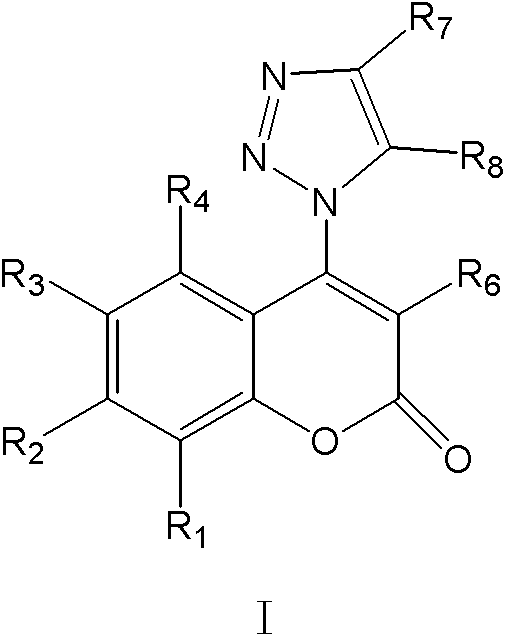
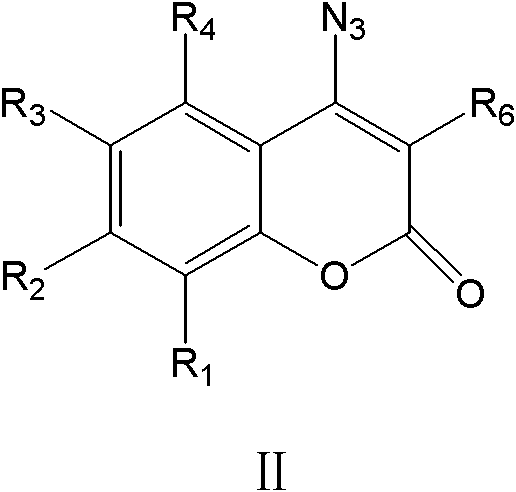
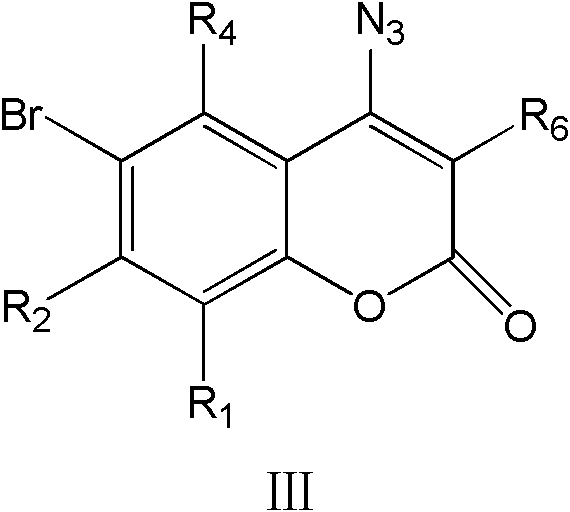
4-1,2,3-triazole-coumarin derivative and its preparation method and application
A technology of coumarin derivatives and triazoles, which is applied in the field of chemical medicine and can solve the problems of poor drug selectivity, restrictions on drug use, and toxic and side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0080] Example 1 Preparation of 4-azido-6-bromocoumarin
[0081] The synthetic route is as follows:
[0082]
[0083] 1, the preparation of p-bromoacetate phenol
[0084]It is prepared from p-bromophenol according to the existing published method, for example: p-bromophenol (10g, 57.8mmol) and acetic anhydride (25ml, 232mmol) are mixed and added to pyridine (22ml). After the addition was complete, the mixture was heated to 100°C for 3.5 hours. After completion of the reaction, cool the reaction, add 150ml of water, acidify with dilute hydrochloric acid, then extract with ethyl acetate, then wash the organic layer with saturated sodium bicarbonate solution until no bubbles are generated. The organic layer was dried with anhydrous sodium sulfate, spin-dried, and dried under reduced pressure at room temperature to obtain a colorless oily liquid (12.1 g, yield 96%).
[0085] Phenyl p-bromoacetate is also commercially available.
[0086] 2, Preparation of 5-bromo-2-hydroxyac...
Embodiment 2
[0099] Example 2 Compound Ia: 4-(4-(((o-methoxyformyl)phenoxy)methyl)-1H-1,2,3-triazole-1-)-6-bromocoumarin preparation of
[0100]
[0101] Dissolve 4-azido-6-bromocoumarin (70mg, 0.26mmol, 1 equivalent) with methyl 4-(3-propynyloxy)benzoate (74.1mg, 0.39mmol, 2.5 equivalents) In tert-butanol (6ml), add 0.5 equivalent of copper powder and 0.5 equivalent of copper sulfate pentahydrate, heat up to 72°C and react overnight. After the reaction was monitored by TLC, it was extracted with dichloromethane, the organic layer was washed with saturated brine, and dried over anhydrous sodium sulfate. The organic layer was concentrated and then column chromatographed to obtain an off-white solid (95 mg, yield 80%) after drying.
[0102] 1 H NMR (CDCl 3 , 400MHz): 4.02(s, 3H), 5.35(s, 2H), 6.44(s, 1H), 6.93(t, J=7.2Hz, 1H), 7.10(d, J=8.0Hz, 1H), 7.31 ~7.38(m, 2H), 7.59(d, J=8.0Hz, 1H), 7.77(d, J=8.8Hz, 1H), 8.10(s, 1H), 8.17(s, 1H)
Embodiment 3
[0103] Example 3 Compound Ib: Preparation of 4-(4-((p-fluorophenoxy)methyl)-1H-1,2,3-triazole-1-)-6-bromocoumarin
[0104]
[0105] Dissolve 4-azido-6-bromocoumarin (70 mg, 0.26 mmol, 1 equivalent) and 3-(p-fluorophenoxy) propyne (59 mg, 0.39 mmol, 1.5 equivalents) in tert-butanol (6ml), add 0.5 equivalent of copper powder and 0.5 equivalent of copper sulfate pentahydrate, and heat up to 65°C to react overnight. After the reaction was monitored by TLC, it was extracted with dichloromethane, the organic layer was washed with saturated brine, and dried over anhydrous sodium sulfate. The organic layer was concentrated, followed by column chromatography and dried to obtain a colorless flaky solid (97 mg, yield 85%).
[0106] 1 H NMR (CDCl 3 , 400MHz): 5.31(s, 2H), 6.57(s, 1H), 7.02(m, J=9.2Hz, 4H), 7.36(d, J=8.4Hz, 1H), 7.58(dd, J=2.0Hz , 8.8Hz, 1H), 8.08(t, J=4.1Hz, 2H)
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com