Small-size doxofylline freeze-dried powder injection and preparation method and production device thereof
A technology of doxofylline and freeze-dried powder injection, which is applied in the direction of freeze-drying transportation, making medicines into special physical or taking forms, powder transportation, etc., which can solve the problems of increasing filling volume, low product output, Increased energy consumption and other issues, to achieve the effect of reducing packaging volume, reducing costs, and reducing energy consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023]
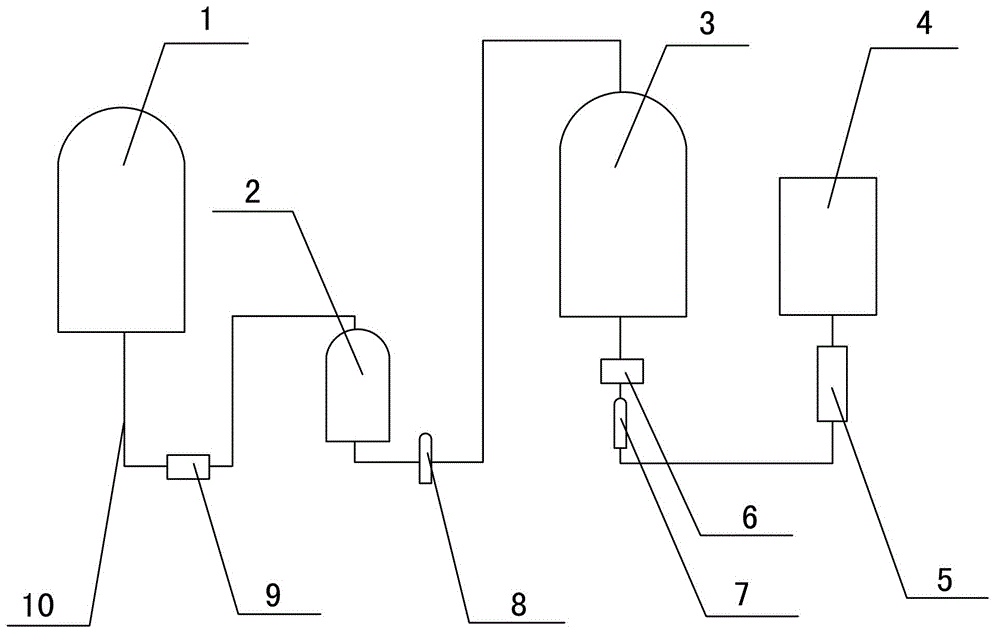
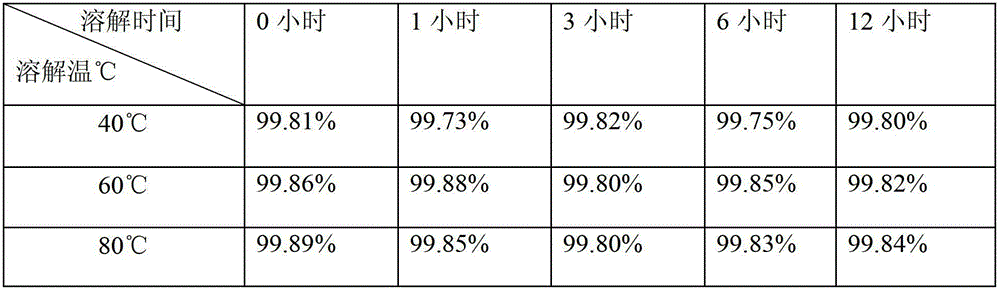
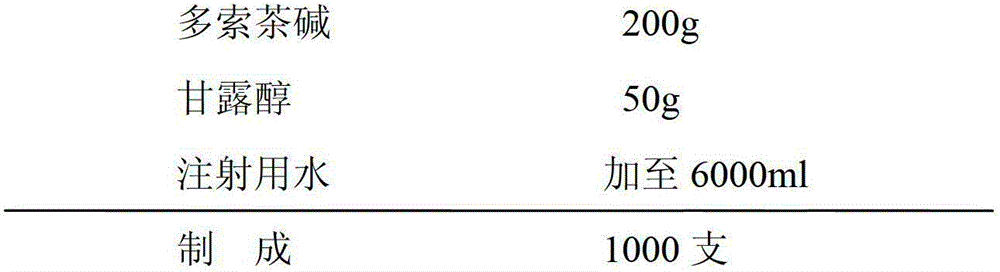
[0024] The preparation method of small-volume doxofylline freeze-dried powder injection is to lower the temperature below 50°C, add mannitol, stir and dissolve, keep the solution temperature between 40°C and 45°C, then add doxofylline, and keep the temperature of the liquid at Between 40°C and 45°C. After the feed liquid is dissolved, take a sample to test the pH value of the feed liquid, which is required to be controlled between 4.5-6.5, and add 0.1% (W / V) activated carbon for decolorization for 30 minutes.
[0025] Turn on the second Grundfos pump 9 and use the titanium rod filter 2 for decarburization and filtration, pass the feed liquid through the second sterilizing filter element 8 to sterilize, and transport it to the aseptic insulation solution storage tank 3 for storage. After sterilizing and filtering, add the remaining amount of water for injection at 40-45°C to the heat-preserving dispensing tank 1, transport it to the titanium rod filter 2 through the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com