Method for preparing 1-carboxylic acid tert-butyl ester-3-fluoro-azetidine derivative
A compound, dicarbonic acid technology, applied in the direction of organic chemistry, can solve the problems of long steps, low yield, incapable of large-scale production, etc., and achieve the effect of mild reaction conditions, simple operation and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
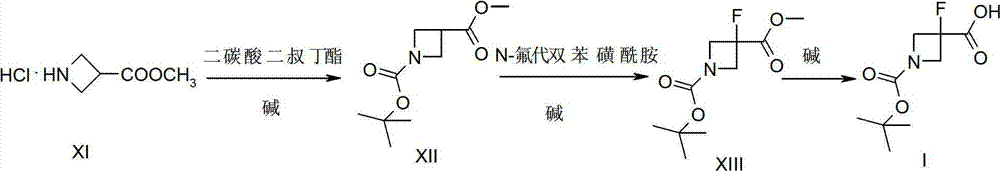
[0018] Preparation of Intermediate Compound XIII
[0019] Synthesis of Compound XII
[0020]
[0021] Add compound XI (740.0g, 4.88mol, 1.0eq.) in 4L water, add 4L ethyl acetate, 2L H 2 O, stirring under ice-water bath, adding NaHCO in batches 3 (820.6g, 9.77mol, 2.0eq.), then add Boc 2 O (1064.8g, 4.88mol, 1.0eq.), stirred at room temperature for 8h, separated after the reaction, extracted once with EA, combined the organic phases, washed with brine, dried over anhydrous sodium sulfate, concentrated to obtain compound XII colorless oil 793.0 g, yield: 75.5%. 1 H NMR (400 MHz, CDCl3) δ (ppm): 4.10-4.12 (d, J=7.6 Hz, 4H), 3.77 (s, 3H), 3.33-3.40 (m, 1H), 1.46 (s, 9H). Synthesis of Compound XIII
[0022]
[0023] Put compound XII (80.0g, 0.37mol, 1.0eq.), N-fluorobisbenzenesulfonamide (NFSI) (175.8g, 0.557mol, 1.5eq.) into a 2L four-neck flask, add THF500mL, after dissolution, Cool down to about -78°C, add LiHMDS (1M, 670mL, 1.8eq.) dropwise, and keep the reaction fo...
Embodiment 2
[0025] Synthesis of Compound I
[0026]
[0027] Put compound XIII (50.0g, 0.214mol, 1.0eq.) into a 1L four-neck flask, add 2M NaOH 214mL and methanol 100mL, react at 20°C for 4 hours, adjust the pH to acidic with 2N HCl, concentrate, and dichloromethane (500mL×3) extraction, the combined organic phases were dried over anhydrous sodium sulfate, and spin-dried to obtain 46.4g of compound I as a white solid, with a yield of 99.0% and a purity of 98%. 1 H NMR (400MHz, CDCl 3 ) δ (ppm): 7.54 (b, 1H), 4.41-4.49 (q, 2H), 4.20-4.28 (q, 2H), 1.48 (s, 9H).
Embodiment 3
[0029] Preparation of Intermediate Compound XIII
[0030] Synthesis of Compound XII
[0031]
[0032] Add compound XI (740.0g, 4.88mol, 1.0eq.) in 4L water, add 4L THF, 2L H 2 O, stirring under ice-water bath, adding Na in batches 2 CO 3 (1553g, 14.655mol, 3.0eq.), then add Boc 2 O (1597.2g, 7.32mol, 1.5eq.), stirred at room temperature for 10h, separated after the reaction, extracted once with EA, combined the organic phases, washed with brine, dried over anhydrous sodium sulfate, concentrated to obtain compound XII colorless oil 740.4 g, yield: 70.5%. 1 H NMR (400 MHz, CDCl3) δ (ppm): 4.10-4.12 (d, J=7.6 Hz, 4H), 3.77 (s, 3H), 3.33-3.40 (m, 1H), 1.46 (s, 9H). Synthesis of Compound XIII
[0033]
[0034] Put compound XII (80.0g, 0.37mol, 1.0eq.), N-fluorobisbenzenesulfonamide (NFSI) (175.8g, 0.557mol, 1.5eq.) into a 2L four-neck flask, add THF500mL, after dissolution, Cool down to about -78°C, add NaHMDS (1M, 745mL, 2.0eq.) dropwise, and keep the reaction for 4h ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com