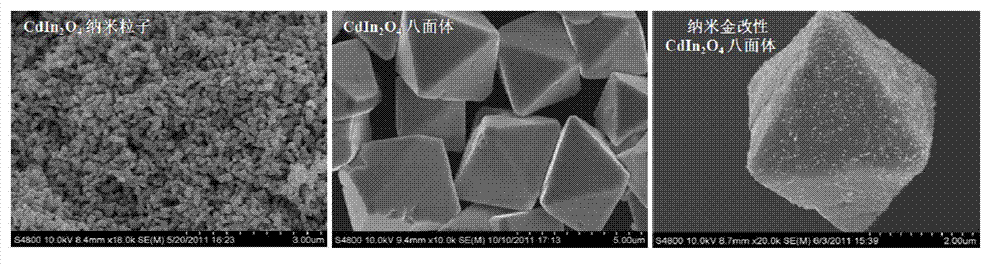
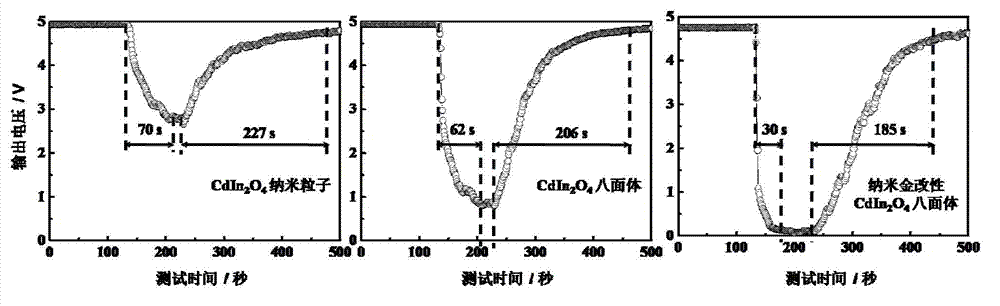
Cadmium indiumate octahedron microcrystal and its preparation method
A technology of octahedron and cadmium indium acid, which is applied in the field of cadmium indium acid octahedral microcrystals and its preparation, can solve the problems of complex process, limited gas-sensing performance of materials, high cost, etc., achieve simple preparation method, improve gas-sensing characteristics, cheap to produce effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Embodiment 1, preparation cadmium indium acid octahedral crystallite
[0026] Add 9 mL of indium sulfate aqueous solution (concentration: 0.4 mol / L) dropwise into 10 mL of cadmium chloride (concentration: 0.2 mol / L) aqueous solution, stir well to obtain a transparent solution; then slowly add 1 mol / L aqueous sodium hydroxide solution Add it dropwise to the above solution, and control the molar ratio of indium sulfate, cadmium chloride and sodium hydroxide to 1.8:1:8 to obtain a white colloidal suspension. Keeping it warm for 15 hours, cooling naturally to obtain a cadmium indium acid precipitate, washing the precipitate with deionized water three times, and drying to obtain a light yellow powder material.
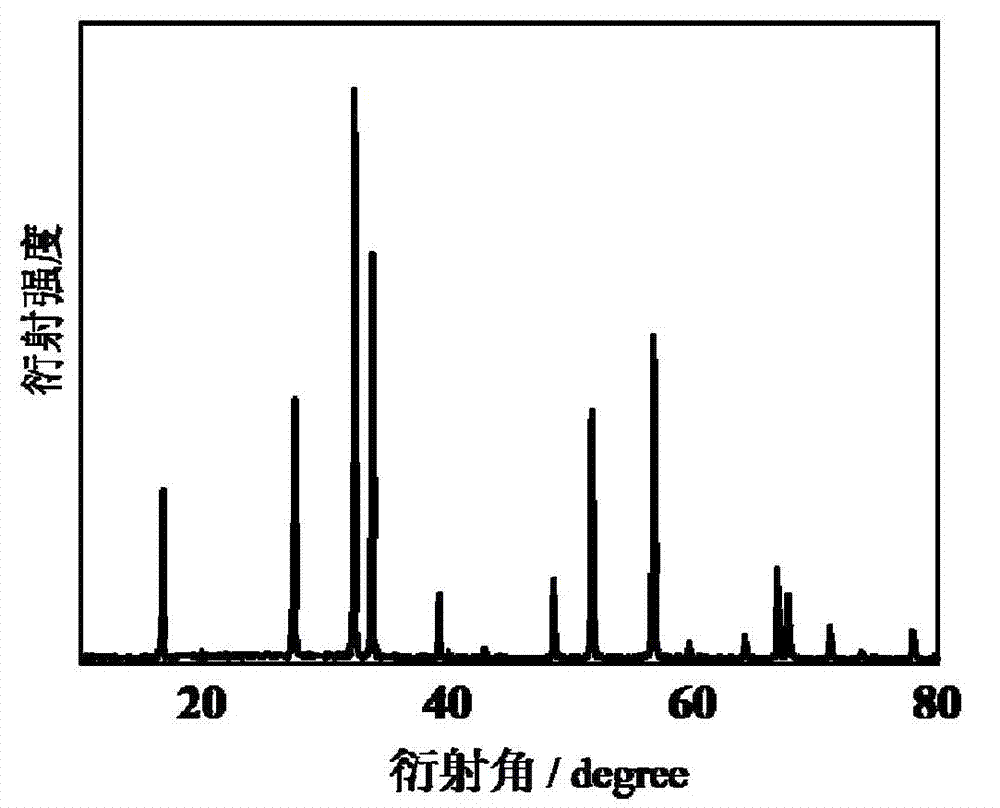
[0027] Its X-ray diffraction pattern is as figure 1 As shown, it can be seen from the figure that the product is a pure-phase cadmium indium crystallite.
Embodiment 2
[0028] Embodiment 2, preparation cadmium indium acid octahedral crystallite
[0029] Add 9mL indium sulfate aqueous solution (concentration: 0.4mol / L) dropwise to 10mL cadmium chloride (concentration: 0.2mol / L) aqueous solution, stir well to obtain a transparent solution; then slowly add potassium hydroxide solution with a concentration of 1mol / L Add it dropwise to the above solution, and control the molar ratio of indium sulfate, cadmium chloride and potassium hydroxide to 1.8:1:8 to obtain a white colloidal suspension. Insulated for 15 hours, cooled naturally to obtain a precipitate, washed five times with deionized water, and dried to obtain a light yellow powder material.
[0030] The XRD ray diffraction pattern of the product and figure 1 Similarly, the product is shown to be phase-pure cadmium indate crystallites.
Embodiment 3
[0031] Embodiment 3, preparation cadmium indium acid octahedral crystallite
[0032] Add 9mL of indium sulfate solution (concentration: 0.4mol / L) dropwise into 10mL of cadmium nitrate (concentration: 0.2mol / L) solution, stir thoroughly to obtain a transparent solution, then slowly drop potassium hydroxide solution with a concentration of 1mol / L Add it to the above solution, control the molar ratio of indium sulfate, cadmium nitrate and potassium hydroxide to 1.8:1:8 to obtain a white colloidal suspension, move the suspension to a reaction kettle, and keep it warm at 260°C for 15 Hours, natural cooling to obtain a precipitate, the precipitate was washed 5 times with deionized water, and dried to obtain a light yellow cadmium indium acid microcrystalline powder material.
[0033] The XRD ray diffraction pattern of the product and figure 1 Similarly, the product is shown to be phase-pure cadmium indate crystallites.
PUM
| Property | Measurement | Unit |
|---|---|---|
| electrical resistivity | aaaaa | aaaaa |
| transmittivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com