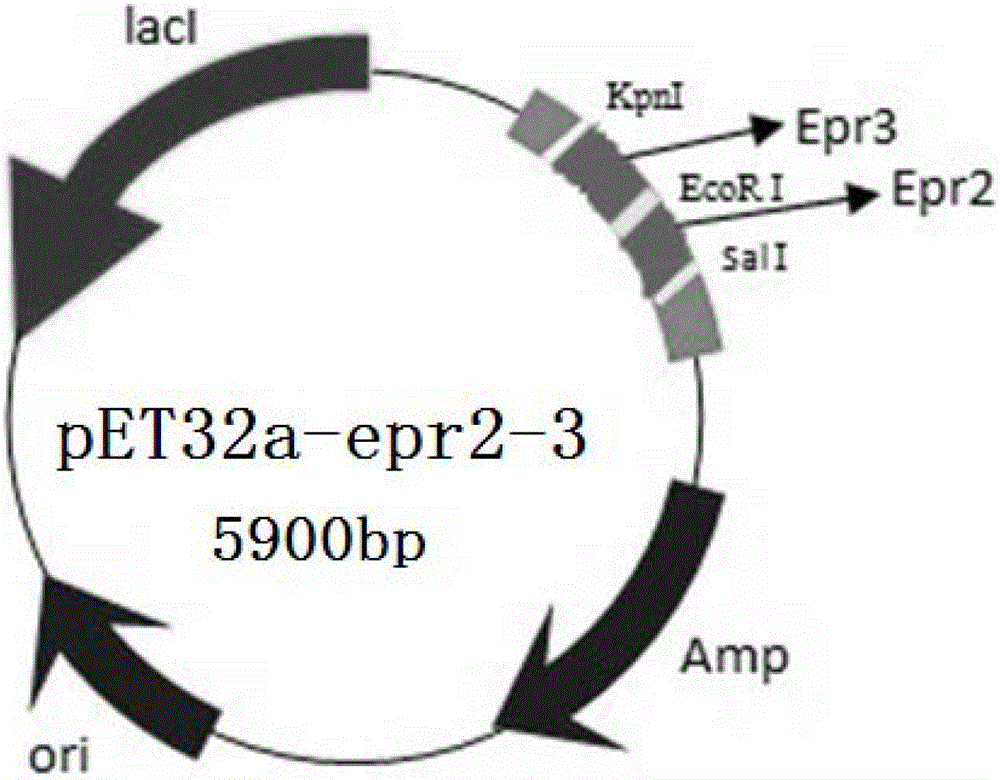
Recombinant plasmid and subunit vaccine of extracellular protease recombinant protein of Aeromonas hydrophila prepared from same plasmid
A technology of Aeromonas hydrophila and extracellular protease, which is applied in the biological field to achieve the effects of simple production process, low requirements for storage and transportation conditions, and broad application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
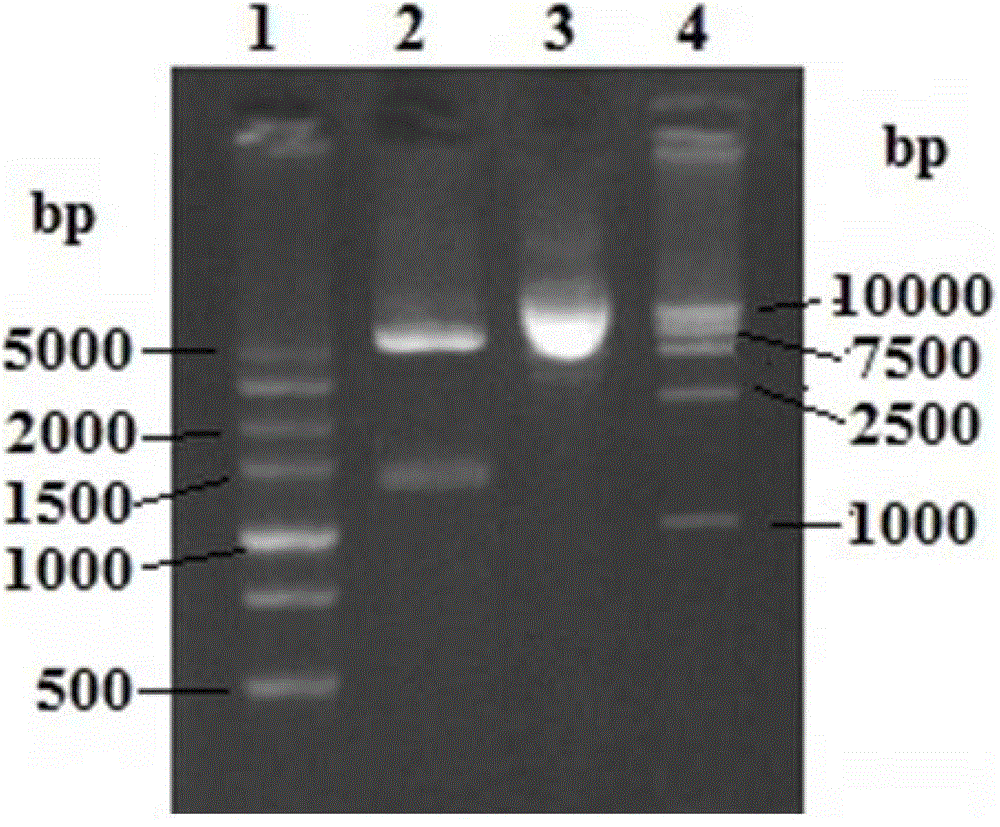
[0038] 1. Amplification, cloning and identification of epr2 and epr3 genes
[0039] 1.1 Design and synthesis of PCR primers
[0040] Design two pairs of specific primers for epr2 (GenBank CP000462.1) and epr3 (GenBank EU275147.1) according to the gene sequence of the Ah standard strain J-1, and contain restriction enzymes at the 5' ends of the primers cut site. Because it was synthesized by Shanghai Invitrogen Biological Company.
[0041] Epr2-1: 5′-CCG GAA TTC ATG ACA CTC TTG CTC ACC ACC C-3′ (SEQ ID No. 1),
[0042] Epr2-2: 5'-GTC GAC CTA GGA GGC GGG CAG CC-3' (SEQ ID No. 2),
[0043] Epr3-1: 5′-GGT ACC ATG AAA GCG ACT CCC ATT GCC C-3′ (SEQ ID No. 3),
[0044] Epr3-2: 5'-CCG GAA TTC TCA GTT TTC GCT CGG CGT ATT CTC-3' (SEQ ID No. 4)
[0045] 1.2PCR
[0046] Take 12.5μl of 2*Taq PCR Mix, epr2-12μl, epr2-22μl, 4μl of Aeromonas hydrophila J-1 strain (CGMCC NO.3220) bacterial solution, and make up to a total volume of 25μl with sterile ultrapure water. Perform the reaction o...
Embodiment 2
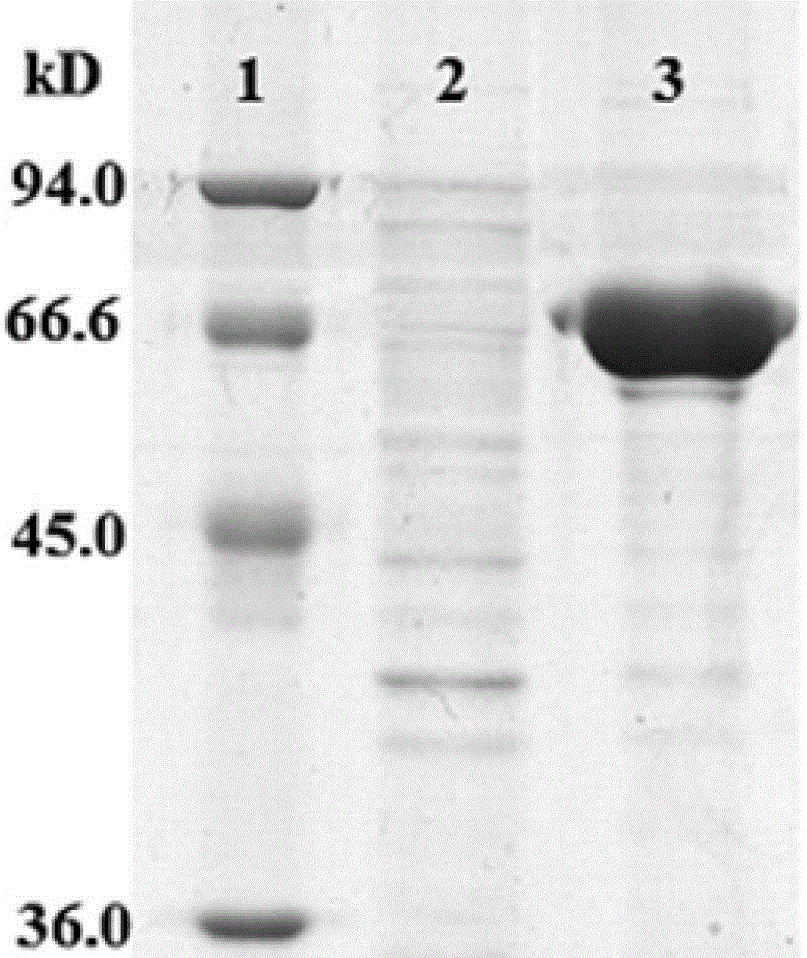
[0062] 2.1 Expression of recombinant protein Epr2-3
[0063] The recombinant Escherichia coli bacterial liquid identified as positive in Example 1 was inoculated into 5 mL of fresh LB liquid medium, and cultured with shaking at 37° C. for 14-16 hours. Take the fresh bacterial solution and re-inoculate it in 200mL fresh LB liquid medium, shake and culture at 200rpm at 37°C for 4 hours, until OD600=0.6; add 0.1M IPTG to the final concentration of 1mmol / L, and continue to shake and culture for 4 hours; take out the bacterial solution and centrifuge in 1.5μL In the tube, centrifuge at 13,000 rpm at 4°C for 2 minutes, resuspend the centrifuged cells with 40 μL PBS (ph=7.2), and add 10 μL of 5×SDS loading buffer; heat and boil to 100°C for 10 minutes, and centrifuge at 13,000 rpm for 2 minutes. Take 10 μL of the lysate for SDS electrophoresis, first at 80V for 30min, then at 120V. After electrophoresis, stain with Coomassie Brilliant Blue G250 for 2 hours, and then decolorize once ...
Embodiment 3
[0069] 3.1 Determination of the median lethal dose of ICR mice with Aeromonas hydrophila J-1 strain
[0070] Eighty four-week-old ICR female mice were randomly divided into 8 groups, 10 in each group. Aeromonas hydrophila J-1 strain (CGMCC NO.3220) was inoculated into LB liquid medium and cultured with shaking at 28° C. to logarithmic growth phase. Collect the bacteria and dilute it with 10mMPBS (ph=7.2) solution to a concentration of 2.5×10 3 CFU / ml. Mice in groups 1-7 were injected with 0.2 mL of bacteria, and the number of deaths was recorded. Group 8 was injected with PBS as the blank control group. This experiment was repeated twice. The results are shown in Table 1.
[0071] Table 1
[0072]
[0073] 3.2 Determination of antibody titer in immunized mouse serum by indirect ELISA
[0074] Forty 4-week-old ICR female mice were randomly assigned to 4 groups, 10 in each group. Group 1 was injected intramuscularly with 100ug / mL recombinant protein Epr2-3 and 0.2mL ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com